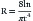Chapter 44 Specific Diseases of the Respiratory System
Upper Airway
where R is the resistance to gas flow, l is the length of the tube, n is the viscosity of the gas, and r is the radius. Unfortunately, airflow through a narrowed trachea is usually turbulent, which worsens the situation because resistance to turbulent flow of gas past an obstruction is inversely proportional to the fifth power of the radius of the lumen.1,2 Gas exchange will be dramatically reduced by minor degrees of impingement on an infant’s trachea. Consequently, a child will not tolerate lesions that would not even produce symptoms in an adult.
Initial Management
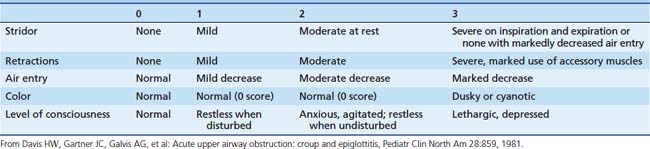
If it is thought to be safe, examination of the patient’s head and neck may reveal the cause of the illness. Depending on the patient’s condition, the degree of respiratory embarrassment may be quantified with a rating scale (Table 44-1). One of the primary benefits of using such a scale is that signs of respiratory obstruction are systematically sought and objectively documented. This information may be valuable in helping to define the course of the patient’s illness and the response to treatment. During the initial evaluation, an assessment may be made regarding the likely location of the obstruction. Extrathoracic airway obstruction usually results in stridor (the obstruction is most severe during inspiratory phase), while intrathoracic airway obstruction usually results in wheezing (the obstruction is most severe during expiratory phase). Localizing the obstruction in this manner helps to narrow the diagnostic possibilities.
The initial evaluation should allow one to make important triage decisions about management and further evaluation of the patient with upper airway compromise. Depending on the severity of the illness, a decision must be made about which diagnostic tests will be undertaken. In the case of severe respiratory compromise, it may be necessary to plan for invasive procedures (endotracheal intubation or operative intervention) while the diagnostic evaluation is being performed. Finally, it should not be forgotten that pulmonary edema might follow relief of severe upper airway obstruction.3 Postobstructive pulmonary edema may be severe enough to require vigorous therapy, including endotracheal intubation, mechanical ventilation, and positive end-expiratory pressure.
Congenital Malformations
Choanal Atresia
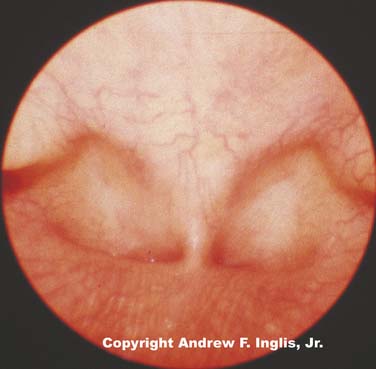
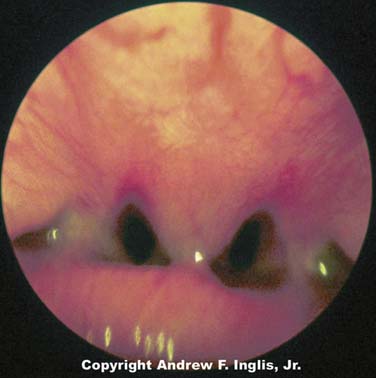
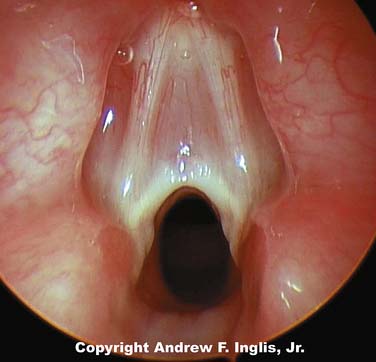
Choanal atresia is estimated to occur about once in every 5000 to 9000 live births.4 Choanal atresia is seen commonly with other defects, especially CHARGE syndrome, which accounts for 25% of all patients with choanal stenosis.5 Unilateral choanal atresia, the most common form, is often seen without accompanying congenital defects and may not be diagnosed at the time of delivery. Bilateral choanal atresia almost always occurs in the presence of other congenital defects.5 For the first 5 months of life, many infants breathe only through their noses and do not open their mouths when the nasal passages are occluded; consequently, bilateral choanal atresia often results in respiratory distress shortly after birth. Bilateral choanal atresia (Figure 44-1) is diagnosed through examination of the naris with the mouth closed. If no airflow is present, a presumptive diagnosis of choanal atresia is established. Some authorities advocate passing a thin, flexible catheter through the naris. This will confirm the diagnosis of choanal atresia; however, if the symptoms are resulting from choanal stenosis, edema formation following even minor trauma of the nasal mucosa after catheter placement may lead to complete occlusion of the nasal airway and worsening of respiratory distress. Surgery is indicated for the correction of bilateral choanal atresia if the infant has symptoms6 (Figure 44-2). Infants with bilateral choanal atresia generally have surgery within the first 3 months of life, while infants with unilateral choanal atresia typically have surgery after the second year of life.7 Topical application of mitomycin to inhibit fibroblast proliferation has been shown to be an effective adjunct to surgical repair of choanal atresia.8 Although choanal atresia is the most common cause of nasal airway obstruction, midline nasal masses such as meningoencephaloceles, gliomas, or dermoid tumors can also cause obstruction. Because these lesions may originate from within the cranial vault, computed tomography (CT) scanning or magnetic resonance imaging (MRI) should be performed before a biopsy or surgical correction of the abnormality is attempted.9
Laryngomalacia
Laryngomalacia is the most common congenital anomaly of the larynx. The infant has inspiratory stridor that is exacerbated by crying or distress. Although no gross anatomic abnormalities are present, the laryngeal cartilages lack their usual rigidity. When the larynx is observed during fiberoptic examination of the glottis, the arytenoid cartilages and supraglottic structures collapse inward (toward the glottis) during inspiration, leading to inspiratory stridor. The negative intrathoracic pressure generated during inspiration contributes to a high incidence of gastroesophageal reflux10 and pulmonary aspiration.11,12
These abnormalities can be graphically observed with fiberoptic laryngoscopy, which shows the dynamic component, with obstruction during inspiration and full airflow during expiration. In some patients with laryngomalacia, gastroesophageal reflux may be the primary cause of the airway compromise, whereas in others it may be a significant cofactor exacerbating preexisting neurologic or anatomic abnormality.13 The respiratory embarrassment associated with this problem is usually minor and self-limited, although hypoxia and hypercapnia have been documented.14 Infants tend to outgrow this problem during the first year of life; however, the condition may be severe enough in some infants that activities such as feeding are compromised. In the most severe cases, surgical intervention may be necessary.15 The goal is to relieve airway obstruction by excision of tissue that collapses into the glottis during inspiration.
Laryngeal Webs, Stenosis, and Tumors
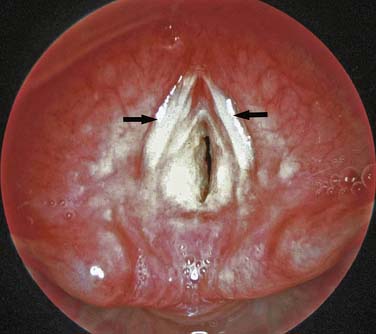
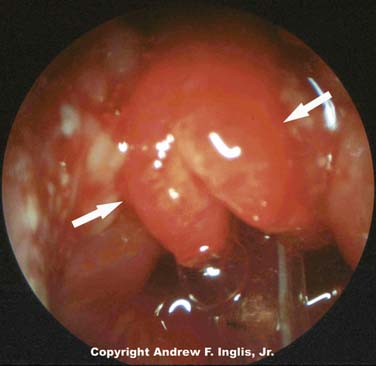
Laryngeal webs usually occur at the level of the glottis and are usually located anteriorly. These may be congenital or acquired and are generally thin membranes of soft tissue that partially occlude the tracheal opening, producing symptoms of feeble cry and dyspnea shortly after birth (Figure 44-3).16 Surgical lysis of these lesions corrects the problem. Laryngeal cysts and laryngoceles are soft tissue masses that protrude into the glottic lumen (Figure 44-4). The resulting respiratory compromise is usually recognized as inspiratory stridor. Treatment is surgical excision of the lesion.17
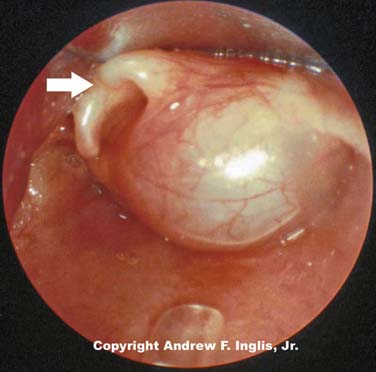
Figure 44–4 A large laryngeal cyst protrudes from the lateral wall of the trachea, just below the level of the glottis.
(Copyright Andrew F. Inglis Jr.)
Another lesion presenting as inspiratory stridor is congenital laryngotracheal (subglottic) stenosis. This is the second most frequent cause of stridor in infants.18 The infant with this problem may have symptoms when newborn but often comes to medical attention later when the tracheal edema produced by a minor respiratory infection causes severe inspiratory stridor. This may be initially diagnosed as croup (laryngotracheobronchitis) but is noted to recur with each subsequent upper respiratory infection. Although the diagnosis of laryngotracheal stenosis may be made radiographically, it is usually established with bronchoscopy. If endotracheal intubation is necessary, a smaller than normal endotracheal tube should be used to reduce trauma and ischemia of the subglottic tissues. Depending on the severity of the lesion, surgical intervention may be necessary (see the discussion about acquired laryngotracheal [subglottic] stenosis for details of the surgical procedures).
Soft tissue masses may reduce the caliber of the tracheal lumen, either by extrinsic compression, as happens with a cystic hygroma, or by growth into the tracheal lumen from the tracheal wall, as happens with a hemangioma. Although these lesions may be present at birth, they often do not produce symptoms for the first few months until the growing lesion further impinges on the trachea. Although surgery is frequently used for treatment of tracheal hemangiomas,19 pioneering work by Judah Folkman was instrumental in demonstrating that some of these lesions respond to steroid therapy.20
Vascular Impingement on the Trachea
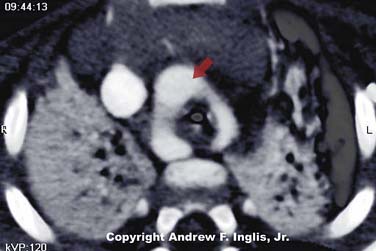
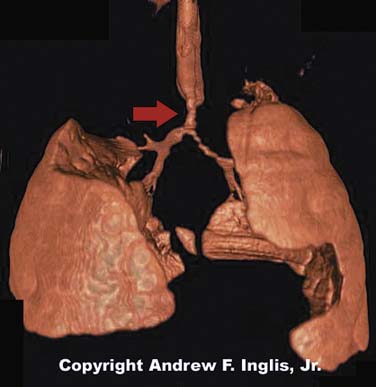
The trachea may be compressed by the presence of an abnormal vascular structure (Figure 44-5). The innominate artery is the most common vessel causing tracheal compression. Vascular rings and enlarged pulmonary arteries are also known to cause tracheal compression, as are a variety of other vascular abnormalities.21 These lesions may present with physical findings such as stridor or wheezing. Alternatively, the patient may be symptom-free, but may suffer respiratory problems such as recurrent lobar atelectasis or frequent pulmonary infections. Because of this, it is difficult to recognize a vascular ring as the underlying cause of illness.22 Careful inspection of the chest radiograph may reveal indentation of the trachea, but often this sign is absent. Barium swallow has been the historic method of diagnosing vascular impingement of the trachea. CT scanning and MRI have become the diagnostic modalities of choice (Figures 44-6 and 44-7).23,24 These noninvasive methods are effective at showing complex three-dimensional cardiovascular anatomy, especially the extracardiac morphology. Treatment involves surgical correction of the vascular anomaly, in severe cases. Respiratory distress may persist postoperatively because prolonged compression of the trachea has made the affected segment softer and collapsible. In severe cases, the tracheomalacia may severely compromise the patient and may be improved by surgical intervention to prevent tracheal collapse.25
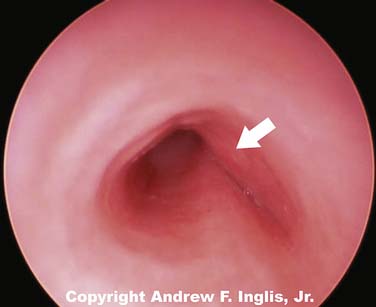
Figure 44–5 The lateral portion of the tracheal lumen is severely compressed by the impingement of the vascular ring.
(Copyright Andrew F. Inglis, Jr.)
Bronchomalacia and Intrathoracic Tracheomalacia
During normal respiration, the upper airway is subject to cycles of positive and negative intraluminal pressure. The cartilaginous components of the upper airway are rigid ringlike structural elements that resist the tendency to collapse caused by the cycling of pressure within the airway lumen. When these structures lack their characteristic rigidity, the mechanics of breathing are altered.26 The symptoms produced by these changes depend on the location of the damaged cartilages. Characteristically, intrathoracic cartilaginous lesions such as bronchomalacia or tracheomalacia impede exhalation. Diagnosis of this problem may be made through observation of collapse of the upper airways during active exhalation, such as occurs while crying. Collapse can be observed with several diagnostic modalities including fluoroscopy, flexible or rigid bronchoscopy, and ultrafast CT scanning.27
Although these lesions may be congenital, many of the cases of tracheomalacia and bronchomalacia seen in the pediatric intensive care unit (PICU) are the result of an infectious or mechanical insult to the trachea. Infants with bronchopulmonary dysplasia and persistent respiratory problems may be affected by bronchomalacia alone or in combination with tracheomalacia.28 The obstructive symptoms produced by these lesions may be relieved by continuous positive airway pressure to maintain patency of the airway during exhalation.29 The level of continuous positive airway pressure necessary to improve respiratory function may be assessed clinically (relief of obstructive symptoms), mechanically (measurement of flow-volume loops), or bronchoscopically (maintenance of airway patency throughout the respiratory cycle).30 With sufficient time, many of these infants outgrow their respiratory difficulties. As an alternative to tracheostomy and positive airway pressure, some have advocated surgical intervention with pericardial flap aortopexy31 or, in extreme situations, metallic airway stents.32
Infectious Processes
Laryngotracheobronchitis
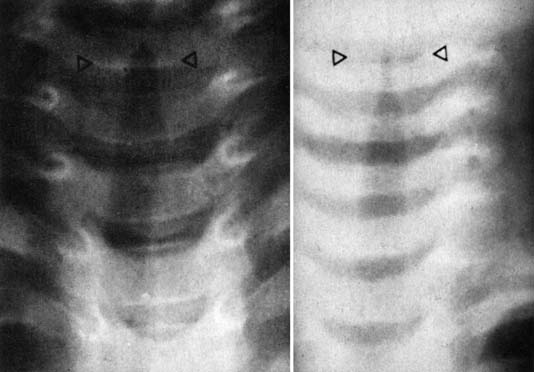
Laryngotracheobronchitis (croup) is a common childhood infection. It is caused by a variety of infectious agents; parainfluenza virus, coronavirus, and rhinovirus are the most common.33 This is a seasonal illness, occurring predominately during winter months, and most commonly affecting children from age 6 months to 3 years. There is frequently a history of prodromal infection accompanied by an unusual cough (described as sounding like the bark of a seal). Swelling of the tracheal mucosa in the subglottic region causes airway compromise (Figure 44-8). Medical attention is usually sought when the child develops inspiratory stridor and respiratory distress. Various scales have been devised to quantify the severity of the stridor to document the progression of the illness and the response to therapy. One of the most commonly employed scales is the Westley scale,34 which has been validated (see Table 44-1).35
When a chest radiograph is obtained during an episode of laryngotracheobronchitis, the trachea is seen to have a gradual progressive narrowing of its lumen, reaching the narrowest point just below the vocal cords (the “steeple sign”) (Figure 44-9). The upper glottis, as seen on a lateral neck radiograph, is normal.
Many care providers believe that exposing the child to cold or misty air often dramatically improves the symptoms; although evidence in support of this therapy is lacking.36,37 When the illness is refractory to these measures, racemic epinephrine has been shown to produce dramatic reduction of airway obstruction. This probably is accomplished by stimulation of the α-adrenergic receptors, producing vasoconstriction and resulting in diminished tracheal edema. Rebound tracheal edema may occur several hours later as the effect of the racemic epinephrine dissipates. Because this problem is unpredictable, the child should be admitted to the hospital for observation after racemic epinephrine has been used.
The practice of treating laryngotracheobronchitis with corticosteroids is widespread, especially for hospitalized patients.38 Oral, intramuscular, and nebulized corticosteroids have been shown to be beneficial in randomized, blinded trials.39,40 Meta-analyses in which the efficacy of corticosteroids was evaluated suggest that corticosteroids reduced the need for endotracheal intubation or inhaled epinephrine, hasten improvement in the first 24 hours of illness, shorten the duration of hospitalization, and reduce the frequency of readmission.41–44
Mixtures of 70% helium and 30% oxygen (heliox) may be beneficial because the characteristics of this mixture permit greater gas flow past areas of airway narrowing. Some authors suggest that this therapy is as efficacious as racemic epinephrine45; however, this therapy has not been conclusively demonstrated to be superior to the administration of supplemental oxygen by itself.46
Later, when an audible leak around the endotracheal tube is present, the trachea may be extubated with a high probability that reintubation will not be necessary.47 If a leak does not become audible after 2 to 4 days, it is our practice to extubate the trachea, because prolonged intubation may increase the risk for subglottic injury. Racemic epinephrine is commonly needed to treat stridor after extubation. If a patient should have especially severe or recurrent laryngotracheobronchitis, an anatomic lesion causing tracheal narrowing should be suspected.
Epiglottitis
Epiglottitis caused by Haemophilus influenzae type B was once a common cause of serious respiratory illness in pediatric patients, but the widespread use of H. influenzae type B vaccine has reduced the frequency of this problem by more than 90% in young children.48 Patients who present with epiglottis are now older, with an average age of 11.6 years, as opposed to an average age of 5.8 years before the advent of Hib vaccination.49 Although cases of H. influenzae epiglottis continue to occur, even among vaccinated patients,50 other causes of epiglottitis have assumed greater importance in the postvaccination era. Group A β-hemolytic Streptococcus is now identified as the cause of epiglottitis in many patients and is clinically indistinguishable from epiglottitis caused by H. influenzae type B.51 Thermal injury to the epiglottis from ingesting hot liquids can also cause epiglottitis.52 Several points serve to distinguish epiglottitis from laryngotracheobronchitis (Table 44-2).
Management of epiglottitis in young children is a multidisciplinary undertaking, involving pediatric intensive care specialists, anesthesiologists, and otolaryngologists. When a child with presumed epiglottitis is admitted to the emergency department, this team should be notified in anticipation of taking the child to the operating room to secure his or her airway. As the team members are being notified, lateral radiographs of the neck may be obtained if tolerated by the patient. This may be done with the child sitting on the parent’s lap to minimize the child’s anxiety. In epiglottitis, the anteroposterior view of the trachea appears normal, but a lateral neck radiograph shows a markedly swollen and edematous epiglottis (Figure 44-10). The diagnostic evaluation of the patient should proceed expeditiously, while care is taken to disturb the patient as little as possible. For this reason, fiberoptic examination of the epiglottis in the awake patient is usually not advisable. Attempts to examine the oropharynx directly or to start an intravenous line should be discouraged. The apprehension caused by these events may lead to tracheal obstruction by the enlarged epiglottis. If the patient will tolerate it, humidified oxygen should be administered, preferably through a plastic hose held by the parent.
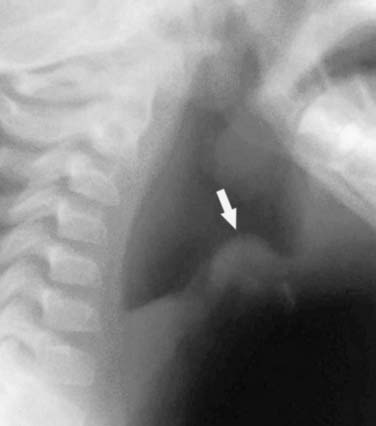
Figure 44–10 Lateral radiograph of the neck of a patient with epiglottitis. Note the large, swollen epiglottis.
If the diagnosis of epiglottitis is strongly suspected or confirmed on the lateral neck x-ray film, the child should go to the operating room as quickly as possible. In the operating room, the patient is anesthetized with an inhaled anesthetic (sevoflurane) and oxygen while the patient is spontaneously breathing. Once the patient has been anesthetized, an intravenous catheter is inserted. Laryngoscopy is then performed (Figure 44-11). It may be exceedingly difficult to obtain a direct view of the glottis and trachea because of the large swollen epiglottis. Nevertheless, it is almost always possible to pass an endotracheal tube through the edematous tissues and into the trachea. Nasotracheal intubation is preferred to orotracheal intubation because the tube is more readily secured to the face, the patient cannot bite the tube, and salivation is decreased. An otolaryngologist should be in the operating room and ready to do an emergency tracheostomy if an airway cannot be secured by endotracheal intubation, although this is rarely necessary. As with laryngotracheobronchitis, endotracheal intubation is preferred to tracheostomy because it has been shown that complications are more common when a tracheostomy has been routinely used to treat epiglottitis. After the airway is secured, blood cultures and cultures of the epiglottis are obtained, and antibiotic therapy is initiated with a penicillinase-resistant antibiotic because of the high incidence of H. influenzae resistance to ampicillin.53
In the PICU, patients usually require endotracheal intubation for 24 to 72 hours while the swollen epiglottis returns to normal size. The patient may be allowed to breathe spontaneously through the endotracheal tube or may undergo mechanical ventilation. Variable amounts of sedation are usually necessary. Extraepiglottic sites of H. influenzae infection are common. In one series, pneumonia occurred in 25% of patients with epiglottitis.54
The management of epiglottitis depends upon the patient’s age. Adults and teenagers with epiglottitis usually present with severe pharyngitis, but usually have mild or absent airway obstruction. In contrast to younger unsedated children, teenagers and adults may tolerate examination of the airway with a small fiberoptic bronchoscope. This procedure may have superior diagnostic sensitivity compared to lateral neck radiographs. Although the management of epiglottitis in young children is almost always accomplished with placement of an endotracheal tube, teenagers and adult patients may be admitted to the hospital for close observation and expectant airway management. Endotracheal intubation is reserved for those patients who develop respiratory compromise.55,56
Peritonsillar Abscess
The initial presentation of peritonsillar abscess may resemble that of epiglottitis. The child usually has a severe sore throat and may also have a muffled voice and drooling. If the abscess is of sufficient size, the child may also experience respiratory distress. Unlike epiglottitis, children with peritonsillar abscess often experience trismus and usually do not have respiratory embarrassment. If the abscess is fluctuant, surgical incision and drainage may be indicated. Although trismus may be of concern in evaluation of the patient for anesthesia, there is usually no anatomic restriction of jaw movement. Once the patient has been anesthetized, the mouth may be easily opened. Extubation is almost always possible after the abscess has been drained, unless there is severe inflammation and swelling extending well beyond the tonsillar bed. Intraoral ultrasound examination has been suggested to be a useful test to differentiate abscess from cellulitis.57 The most commonly encountered microorganism is Group A Streptococcus.58
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree