| Distributive | Severe inflammatory response syndrome (SIRS) and sepsis, neurogenic, anaphylaxis, adrenal insufficiency/Addisonian crisis, drug or toxin reaction, hepatic failure |
| Hypovolemic | Hemorrhage (trauma, GI bleed, ruptured AAA), GI losses (diarrhea, vomiting, fistula), insensible losses, third spacing (pancreatitis, burns) |
| Cardiogenic | Myocardial infarction, myocarditis, arrhythmia, cardiac contusion, valve dysfunction, thyrotoxicosis, end-stage cardiomyopathy |
| Obstructive | Tension pneumothorax, cardiac tamponade, pulmonary embolism (PE), constrictive pericarditis, aortic coarctation, excessive PEEP or auto-PEEP |
Presentation
- A patient in shock presents with a wide variety of signs and symptoms related to both the precipitating event and the resultant cellular dysfunction.
- On presentation to the ED, a patient in shock may be very ill and have difficulty giving a history, which may necessitate obtaining collateral information from emergency medical services personnel, family, or friends.
- In general, a patient will appear toxic, in distress, with pale, clammy skin, with tachypnea, and with tachycardia as a result of the body’s stress response to injury.
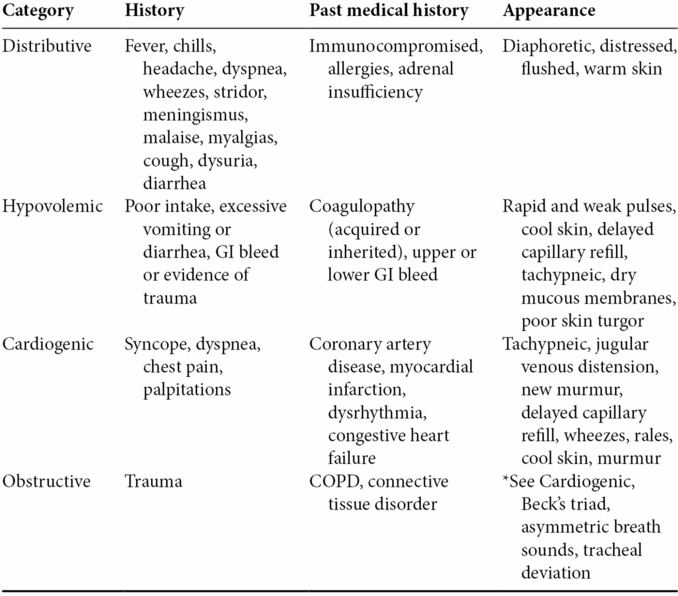
- Subtle clues from the patient’s history including past medical conditions, preceding events, and appearance can help clinicians categorize a patient’s shock state (Table 1.2).
- Patients often present in early shock, which will quickly progress from compensated to decompensated, and finally, refractory or irreversible shock.
- A commonly held misbelief that often leads to delayed treatment and poorer outcomes is that shock necessitates hypotension.
- Compensated or cryptic shock patients may appear relatively normal and asymptomatic as compensatory mechanisms resulting in tachycardia or vasoconstriction have yet to be overwhelmed.
- Decompensated shock patients often appear ill, pale, diaphoretic, tachypneic, tachycardic, and with altered mental status.
- The critical state refractory shock is recognized by manifestations of MODS such as obtundation or coma, refractory hypotension, renal failure, disseminated intravascular coagulation (DIC), and the acute respiratory distress syndrome (ARDS).
- A commonly held misbelief that often leads to delayed treatment and poorer outcomes is that shock necessitates hypotension.
Table 1.2. Presentations of shock
Diagnosis and evaluation
- Vital signs are nonspecific.
- Any single vital sign in isolation is not helpful in diagnosing shock or the possible etiology of shock.
- Shock should be suspected when patients present with a constellation of signs including ill-appearance, tachycardia, tachypnea, hypotension, and oliguria.
- Tachycardia is seen in the hyperdynamic state of shock. However, bradycardia may also be present in the setting of drug overdose (e.g., beta-blockers, calcium channel blockers, digoxin).
- Hypotension is usually a late finding in a previously healthy individual, and noninvasive blood pressure monitoring can be inaccurate. Also, normotension in a previously hypertensive patient can be indicative of shock.
- Any single vital sign in isolation is not helpful in diagnosing shock or the possible etiology of shock.
- Signs of shock are a result of the compensatory mechanisms, organ dysfunction, and the precipitant etiology. They can be helpful in providing clues to the cause of shock.
- Warm extremities can be caused by vasodilation present in distributive shock.
- Cold extremities can be caused by vasoconstriction present in hypovolemic or cardiogenic shock.
- Jugular venous distension in the setting of shock can be caused by cardiogenic or obstructive shock.
- Low jugular venous pressure is indicative of hypovolemic shock.
- Other findings will be present in specific causes of shock:
- Absent breath sounds in tension pneumothorax
- Muffled heart sounds in cardiac tamponade
- Absent breath sounds in tension pneumothorax
- Signs of organ dysfunction include oliguria/anuria, encephalopathy and hypoxia.
- Warm extremities can be caused by vasodilation present in distributive shock.
- Laboratory tests:
- A complete blood count will identify leukocytosis or bandemia in the setting of SIRS. It will also identify anemia (Hct <30% or Hgb <10). However, a normal value may be misleading in the acute stage of blood loss.
- Serum chemistry will assess renal function, hydration status, and detect electrolyte derangements.
- Cardiac biomarkers will identify myocardial injury.
- Lactate and base deficits are markers for tissue hypoperfusion.
- Arterial or venous blood gases will identify oxygenation or ventilation disorders and severe acid–base disturbances.
- Urine or serum hCG (human chorionic gonadotropin) should be obtained in female patients of child-bearing age to evaluate for potential ruptured ectopic pregnancy as a source of hemorrhage and shock.
- A complete blood count will identify leukocytosis or bandemia in the setting of SIRS. It will also identify anemia (Hct <30% or Hgb <10). However, a normal value may be misleading in the acute stage of blood loss.
- Electrocardiogram (ECG):
- Useful for the early diagnosis of acute coronary syndromes, arrhythmias, or electrolyte disturbances.
- Imaging:
- Chest radiography may show edema, effusion, consolidation, pneumothorax, or an enlarged mediastinum and cardiac silhouette.
- Pelvic radiography as a screening tool in blunt trauma may reveal a clinically significant pelvic fracture as a source of hemodynamic instability.
- Point-of-care ultrasonography (US) can be very useful in the management of undifferentiated shock.
- The Focused Assessment with Sonography in Trauma (FAST) examination can quickly identify free fluid in the abdomen as a potential source of bleeding in a hemodynamically unstable patient.
- Cardiac views can reveal pericardial effusion and tamponade physiology.
- Bedside echocardiography can also be used to assess for globally reduced ventricular function, a severely enlarged right ventricle (RV), or preload responsiveness by evaluating the inferior vena cava (IVC).
- The extended FAST examination with lung views may reveal a pneumothorax or pleural effusion.
- Additionally, bedside US of the abdomen can identify a ruptured abdominal aortic aneurysm (AAA) as the cause of shock.
- The Focused Assessment with Sonography in Trauma (FAST) examination can quickly identify free fluid in the abdomen as a potential source of bleeding in a hemodynamically unstable patient.
- Chest radiography may show edema, effusion, consolidation, pneumothorax, or an enlarged mediastinum and cardiac silhouette.
- Conventional echocardiography (transthoracic or transesophageal) will identify ventricular dysfunction, regional wall motion abnormalities, valvular pathology, aortic pathology, tamponade physiology, or RV strain pattern suggestive of massive pulmonary embolism or other causes of right heart failure.
- Computed tomography (CT) scanning may be helpful in identifying the source of shock in specific cases such as pulmonary embolism, aortic dissection, intra-abdominal sepsis, or intra-abdominal hemorrhage.
- Invasive hemodynamic monitoring by way of an arterial line, central venous catheter, or pulmonary artery catheter can further differentiate shock categories by determining values such as mean arterial pressure (MAP), central venous pressure (CVP), pulmonary capillary wedge pressure (PCWP), cardiac output (CO), cardiac index (CI), systemic vascular resistance (SVR), and oxygen transport variables. Different categories of shock have different hemodynamic profiles (Table 1.3).
- Central venous catheter: a catheter placed into a central vein, either the internal jugular, subclavian, or femoral vein. It allows one to administer vasoactive medications and obtain the following measurements (a femoral line does not allow for accurate measurements):
- CVP is an indicator of volume status and cardiac pump function. The target range in septic patients is 8–12 mmHg.
- Central venous oximetry: the ScvO2 value is an indicator of tissue oxygenation and utilization. It is obtained by sampling blood from the superior vena cava (SVC) via a central venous catheter. Low central venous oxygen saturation (<70%) suggests that tissues are extracting more oxygen because of hypoperfusion and hypoxia.
- Arterial line: an invasive catheter placed in the artery (common sites are radial or femoral) that allows for direct measurement of arterial blood pressure. It is more accurate for determining MAP and is helpful in a patient who requires multiple blood draws.
- Pulmonary artery catheter (PAC): an invasive catheter placed in the pulmonary artery that allows a clinician to directly measure PCWP (surrogate for left atrial pressure) and pulmonary artery pressure to assess pump function. It also allows one to measure other hemodynamic parameters including CO, CI, and SVR. The use of a PAC has not been shown to provide a mortality benefit. This procedure is almost never performed in the emergency department.
- CVP is an indicator of volume status and cardiac pump function. The target range in septic patients is 8–12 mmHg.
- Central venous catheter: a catheter placed into a central vein, either the internal jugular, subclavian, or femoral vein. It allows one to administer vasoactive medications and obtain the following measurements (a femoral line does not allow for accurate measurements):
Table 1.3. Differentiating categories of shock
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




