| Cardiovascular disease |
| Arrhythmia |
| Electrolyte abnormality |
| Pulmonary embolus |
| Hypoxic arrest |
| Tension pneumothorax |
| Trauma |
| Cardiac tamponade |
| Hemorrhage/hypovolemia |
| Medication or drug overdose |
| Acidosis |
| Hypothermia |
Diagnosis and evaluation
- An immediate assessment of a patient after the return of spontaneous circulation should include a focused history (usually obtained from bystanders or emergency medical services personnel), physical examination, diagnostic testing, and imaging studies.
- The physical examination should follow the ABCs, checking (1) the airway for appropriate endotracheal tube (ETT) placement, (2) the presence of bilateral breath sounds, (3) circulatory status and blood pressure, (4) heart rate and rhythm, (5) disability with neurological response and Glasgow coma scale, and (6) exposure to fully expose the patient and complete the examination.
- Electrocardiogram (ECG)
- Cardiovascular disease is the most common cause of cardiac arrest; therefore, an ECG should be performed as soon as possible after ROSC to look for evidence of myocardial infarction.
- If acute myocardial infarction is thought to have occurred, an emergency cardiac catheterization should be performed.
- Other abnormalities on the ECG such as T-wave changes suggesting electrolyte abnormalities or ischemia, interval changes concerning for complete heart block, or prolonged QTc should also be promptly addressed.
- Cardiovascular disease is the most common cause of cardiac arrest; therefore, an ECG should be performed as soon as possible after ROSC to look for evidence of myocardial infarction.
- Imaging studies
- Chest radiography: A chest radiograph (CXR) is taken to determine ETT position and to assess for potential etiologies of cardiac arrest such as congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), cardiac tamponade, aortic dissection, or tension pneumothorax. Certain complications of cardiac arrest such as aspiration or the acute respiratory distress syndrome (ARDS) can also appear on the initial CXR.
- Echocardiography: When the diagnosis of cardiac disease is uncertain, a bedside echocardiogram may help detect wall motion abnormalities, assess left ventricular (LV) function, and rule out cardiac tamponade.
- CT scan: A CT scan can be performed to exclude a primary intracranial process, or to evaluate the chest, abdomen, and pelvis.
- Chest radiography: A chest radiograph (CXR) is taken to determine ETT position and to assess for potential etiologies of cardiac arrest such as congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), cardiac tamponade, aortic dissection, or tension pneumothorax. Certain complications of cardiac arrest such as aspiration or the acute respiratory distress syndrome (ARDS) can also appear on the initial CXR.
- Laboratory studies
- Basic serum electrolytes, complete blood count, arterial blood gas, serum troponin, serum lactate, and specific toxicological studies should be drawn as part of the patient workup.
Critical management
- The extent of brain injury and cardiovascular instability are the major determinants of mortality after cardiac arrest.
- Brain injury is responsible for mortality in 68% of out-of-hospital arrests and 23% of in-hospital arrests.
- Perfusion should be optimized early and aggressively.
- Therapeutic hypothermia should then be considered in comatose patients who do not have contraindications for its initiation.
- Hemodynamic optimization
- The systolic blood pressure (SBP) should be kept above 90 mmHg or the mean arterial pressure (MAP) above 65 mmHg.
- Treat hypotension with intravenous fluid (IVF) boluses.
- Inotropic and vasopressor agents such as dopamine and norepinephrine may be used through a central access if the patient is refractory to IVF.
- If ventricular fibrillation (VF) or ventricular tachycardia (VT) preceded the cardiac arrest, an antiarrhythmic agent such as amiodarone may be started.
- Patients should be placed on continuous telemetry with serial 12-lead ECGs performed.
- The systolic blood pressure (SBP) should be kept above 90 mmHg or the mean arterial pressure (MAP) above 65 mmHg.
- Pulmonary optimization
- An endotracheal tube should be placed in unconscious patients to protect the airway. If cardiopulmonary resuscitation is ongoing, care should be taken to avoid interruption of quality chest compressions for the purpose of endotracheal tube placement. A temporary device such as an extraglottic airway may be temporarily placed until ROSC is achieved.
- Titrate the minute ventilation on the ventilator to keep the end-tidal carbon dioxide (PetCO2) at 35–40 mmHg, or the partial pressure of carbon dioxide (PaCO2) at 40–45 mmHg.
- Hypocarbia can potentially cause cerebral vasoconstriction leading to decreased cerebral perfusion and neurological injury.
- FiO2 should also be titrated down to keep the oxygen saturation (SpO2) at 94% or above in order to reduce the potential for oxygen toxicity.
- An endotracheal tube should be placed in unconscious patients to protect the airway. If cardiopulmonary resuscitation is ongoing, care should be taken to avoid interruption of quality chest compressions for the purpose of endotracheal tube placement. A temporary device such as an extraglottic airway may be temporarily placed until ROSC is achieved.
- Neurological protection
- Hypothermia has been shown to significantly improve meaningful neurological outcomes for patients who remain comatose after cardiac arrest from shockable rhythms (VF and VT).
- “Comatose” has been generally defined as a lack of meaningful response to verbal commands.
- Patients who remain comatose after ROSC should be cooled to 32–34°C (89.6–93.2°F) for 12–24 hours.
- Hyperpyrexia should be avoided in these patients as it has been shown to exacerbate the extent of brain damage.
- Hypothermia has been shown to significantly improve meaningful neurological outcomes for patients who remain comatose after cardiac arrest from shockable rhythms (VF and VT).
Hypothermia after cardiac arrest
- Contraindications to cooling
- The only absolute contraindications for therapeutic hypothermia in comatose patients are a do not resuscitate (DNR) order or arrest secondary to traumatic injury.
- Relative contraindications include active bleeding, pregnancy, and septic shock as a cause of the arrest.
- Induction of therapeutic hypothermia can begin before cardiac catheterization and safely be continued throughout the procedure.
- The only absolute contraindications for therapeutic hypothermia in comatose patients are a do not resuscitate (DNR) order or arrest secondary to traumatic injury.
- Cooling methods and monitoring
- There are multiple methods of cooling and none have been proven to be superior.
- Internal systems using intravascular catheters and surface cooling devices are common.
- Cooling blankets and ice bags can be used but patients should be monitored closely as the rate of cooling and the goal temperature cannot be programmed.
- Intravascular cooling with cool IVF is an easy way to initiate cooling in the prehospital setting or in the emergency department: 30 mL/kg of 4°C (39°F) isotonic saline is administered through peripheral access.
- A patient’s core temperature should always be monitored through an esophageal thermometer, pulmonary artery catheter, or bladder catheter.
- The goal temperature should ideally be reached within 6 hours and maintained for 12 to 24 hours.
- There are multiple methods of cooling and none have been proven to be superior.
- Sedation and paralytics
- Sedation and paralytics are used to prevent shivering and decrease agitation, pain, and anxiety, during therapeutic hypothermia.
- Shivering can increase temperature and delay reaching the hypothermia goal.
- Sedation is most commonly provided with propofol or midazolam infusions.
- Midazolam may cause less hypotension than propofol but its effects are longer lasting, which can affect neurological examinations.
- Sedation and paralytics are used to prevent shivering and decrease agitation, pain, and anxiety, during therapeutic hypothermia.
- Rewarming
- During the rewarming phase, the core temperature should be raised gradually by 0.2 to 0.5°C an hour until the goal of 37°C is reached.
- Rapid rewarming can cause electrolyte abnormalities, cerebral edema, and seizures.
- During the rewarming phase, the core temperature should be raised gradually by 0.2 to 0.5°C an hour until the goal of 37°C is reached.
Common complications from cooling
- Seizures
- Continuous electroencephalogram (EEG) monitoring should be used on all patients requiring neuromuscular blockade in order to monitor for seizures.
- If seizures occur, propofol, midazolam, phenytoin, and phenobarbital can be used and are similarly effective. Multiple medications may be required.
- Continuous electroencephalogram (EEG) monitoring should be used on all patients requiring neuromuscular blockade in order to monitor for seizures.
- Coagulopathy
- Hypothermia impairs coagulation and therefore any active bleeding should be controlled before the initiation of cooling.
- In the event of significant bleeding; a significant drop in hemoglobin, hemodynamic instability, intracranial hemorrhage, or noncompressive bleeding, therapeutic hypothermia should be stopped and the patient rewarmed to at least 35°C.
- Hypothermia impairs coagulation and therefore any active bleeding should be controlled before the initiation of cooling.
- Increased risk of infection
- When hypothermia is continued for longer than 24 hours, it has been shown to increase the risk of infection due to decreased leukocyte function.
- Concern for sepsis and septic shock as the cause of arrest is a relative contra-indication to the initiation of hypothermia.
- When hypothermia is continued for longer than 24 hours, it has been shown to increase the risk of infection due to decreased leukocyte function.
- Arrhythmias
- Hypothermia can lead to arrhythmias such as bradycardia and QT interval prolongation.
- If the blood pressure remains adequate then the bradycardia does not need to be addressed.
- Hypothermia can lead to arrhythmias such as bradycardia and QT interval prolongation.
- Hyperglycemia
- Hyperglycemia due to insulin resistance has been shown to result in worse neurological outcomes and increased mortality in post–cardiac arrest patients. However, intense glucose control can also lead to episodes of hypoglycemia and adverse outcomes.
- Glucose control should therefore aim to maintain glucose between 140 and 180 mg/dL (7.77–9.99 mmol/L).
- Hyperglycemia due to insulin resistance has been shown to result in worse neurological outcomes and increased mortality in post–cardiac arrest patients. However, intense glucose control can also lead to episodes of hypoglycemia and adverse outcomes.
- Electrolyte abnormalities
- Hypothermia can lead to increased urine output, a condition known as “cold diuresis,” which in turn can lead to hypovolemia and electrolyte abnormalities.
- Fluid balance should be continuously monitored in all patients and electrolytes measured every 3–4 hours and replaced as needed.
- Hypothermia can lead to increased urine output, a condition known as “cold diuresis,” which in turn can lead to hypovolemia and electrolyte abnormalities.
Special circumstances
- In post–cardiac arrest patients with arrest secondary to known or suspected pulmonary embolism, fibrinolytics should be considered.
- If adequate hemodynamic stability cannot be achieved through IVF and inotropic support, placement of an intra-aortic balloon pump may be necessary.
- If these therapies fail, a left ventricular assist device, or extracorporeal membrane oxygenation (ECMO), may be considered.
Critical care considerations
Vasopressor of choice: Patients who are hypotensive post cardiac arrest from a shockable rhythm (VT or VF) are likely suffering from a degree of cardiogenic shock. The choice of inotrope and/or vasopressor will depend on the patient’s systolic blood pressure (SBP):
- SBP
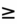 80 mmHg: dobutamine
80 mmHg: dobutamine
- SBP <80 mmHg: dopamine
- SBP <70 mmHg: norepinephrine.
In patients with ROSC post cardiac arrest with pulseless electrical activity (PEA), the choice of inotrope and/or pressor will depend on the etiology of the arrest.
References
Arrich J, Holzer M, Havel C, et al. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2012 Sep 12; 9: CD004128.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






