82 Severe Heart Failure
Heart failure is a very common condition with high mortality and morbidity rates. Data from the Framingham heart study suggest that at 40 years of age, the lifetime risk for congestive heart failure is 21.0% (95% confidence interval [CI], 18.7%-23.2%) for men and 20.3% (95% CI, 18.2%-22.5%) for women.1 The prevalence of heart failure is between 2% and 3% and reaches 10% and 20% in those older than 70 years.2 In the United States, approximately 5 million patients have heart failure, with over 550,000 new cases diagnosed each year.3 Despite improvements in treatment, the overall prevalence of heart failure is increasing because of the aging of the population and better survival following myocardial infarction (MI).3,4 Total heart failure–related costs were about $28 billion in 2005 and consume approximately 2% of national expenditure on health in Europe.5 Patients with severe heart failure often present in extremis, and their condition may deteriorate rapidly, so a sound knowledge of immediate treatment is vital for critical care and emergency physicians. Such patients often respond rapidly to appropriate treatment, making this a very satisfying condition to treat. However, it is important to note that outlook remains poor despite initial clinical improvement.
 Etiology
Etiology
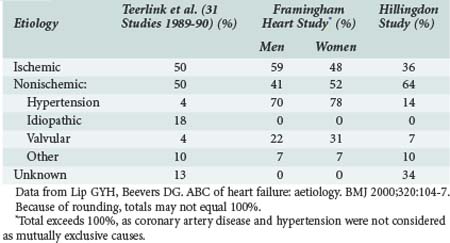
Ischemic heart disease is the most common cause of heart failure, commonly related to previous MI. Although epidemiologic surveys such as the Framingham study suggest a high prevalence of hypertension as the “cause” of heart failure, it is likely that associated ischemic heart disease or arrhythmias also contribute. Other studies have demonstrated similar findings (Table-82-1).
Ischemic Heart Disease
Ischemic heart disease is the most common cause of heart failure in the Western world. Many patients presenting with severe heart failure will give a history of previous MI. However, an episode of severe heart failure may also be the first manifestation of ischemic heart disease, either due to massive MI causing cardiogenic shock6 or as a result of previous silent (or unreported) episodes of ischemia/infarction. It is therefore important to exclude MI in all patients presenting with severe heart failure. Additionally, once the patient is stabilized, adequate secondary preventive strategies are vital to prevent further ischemia or infarction. Some patients with ischemic cardiomyopathy may show evidence of “hibernation” of segments of myocardium,7 and cardiac function in these patients may improve with revascularization (see later discussion).
Dilated Cardiomyopathy
Dilated cardiomyopathy is defined as left ventricular dysfunction of unknown cause. It is therefore a diagnosis of exclusion, and a firm diagnosis of dilated cardiomyopathy can only be made in the presence of a normal coronary angiogram. Intensive investigation of patients with a label of dilated cardiomyopathy may yield a definite cause in at least 50% of cases (Table 82-2). As many as 30% of patients with dilated cardiomyopathy may have a genetic cause of the disease.8
TABLE 82-2 Final Diagnoses in 1230 Patients with Initially Unexplained Cardiomyopathy
| Diagnosis | Number | Percentage |
|---|---|---|
| Idiopathic dilated cardiomyopathy | 616 | 50 |
| Myocarditis | 111 | 9 |
| Ischemic heart disease | 91 | 7 |
| Infiltrative cardiomyopathy | 59 | 5 |
| Peripartum cardiomyopathy | 51 | 4 |
| Hypertension | 49 | 4 |
| Human immunodeficiency virus infection | 45 | 4 |
| Connective tissue disease | 39 | 3 |
| Substance abuse | 37 | 3 |
| Familial | 25 | 2 |
| Valvular disease | 19 | 1.5 |
| Doxorubicin therapy | 15 | 1 |
| Endocrine disorder | 11 | 1 |
| Others | 62 | 5.5 |
Data from Felker GM et al. Underlying causes and long term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000;342:1077-84.
Diabetes
In addition to the role of diabetes as a risk factor for the development of ischemic heart disease and resultant heart failure, there is evidence for a distinct diabetic cardiomyopathy.8 Current guidelines recognize diabetes as a risk factor for heart failure.8 Hemoglobin A1c levels have been shown to be an independent progressive risk factor for cardiovascular and total mortality and rehospitalization rate in heart failure patients.9,10 All patients presenting with heart failure should be screened for diabetes, both for this reason and so that appropriate secondary prevention can be instituted.
Other Possible Causes or Exacerbating Factors
Patients with a long history of stable heart failure may decompensate as the result of a number of different factors. Intercurrent infection is a common cause of decompensation, and prompt recognition and treatment are important. Arrhythmias are also a common cause for decompensation of a previously stable heart failure patient, and a recent meta-analyses of 16 randomized clinical trials (53,969 patients) revealed that the presence of AF is associated with an adverse effect on total mortality, with an odds ratio of 1.40 (95% CI, 1.32-1.48).11 Another common cause of decompensation is anemia, which is often poorly tolerated in patients with heart failure. A meta-analysis of 34 studies comprising 153,180 patients, of whom 37.2% were anemic, showed that adjusted mortality risk of anemia had a hazard ratio of 1.46 (95% CI, 1.26-1.69).12 Poor prognosis associated with presence of atrial fibrillation or anemia was irrespective of left ventricular systolic function, either preserved or impaired.11,12 It is vital to consider and treat such exacerbating conditions where appropriate.
 Presentations of Severe Heart Failure
Presentations of Severe Heart Failure
Acute Presentation: Pulmonary Edema
Cardiovascular Examination
Examination of the pulse may reveal atrial fibrillation. Patients in sinus rhythm are usually tachycardic, although patients with a history of ischemic heart disease may well be taking beta-blockers, which mask tachycardia. Heart rate appears to be an independent and powerful factor of prognosis in heart failure. In the BEAUTIFUL study, heart failure patients with heart rates of 70 bpm or greater had 34% higher risk for cardiovascular death and 53% higher risk admission to hospital than those with heart rate blow 70 bpm. The study has shown 8% and 15% increments of cardiovascular death and hospital admission for every increase of 5 bpm.13
Subacute Presentation: Shortness of Breath/Peripheral Edema
Many patients with severe heart failure present less acutely with varying combinations of breathlessness and edema. This is often the case in patients with a previous diagnosis of heart failure and can be precipitated by intercurrent infection or withdrawal of diuretic or other medication (by the patient or a physician). In the early stages, edema may be more prominent unilaterally, and this may result in diagnostic difficulty. Such patients may be referred for exclusion of deep venous thrombosis (and it is important to be aware that the two conditions can coexist). These patients often report gradually increasing breathlessness with symptoms of orthopnea (shortness of breath occurring when lying supine) and paroxysmal nocturnal dyspnea (sudden shortness of breath waking the patient from sleep). Patients may resort to sleeping in a chair, leading to additional gravitational edema. Edema of the bowel can lead to reduced appetite, so called “cardiac cachexia,” and further edema from hypoproteinemia. Peripheral edema is therefore often multifactorial in patients with heart failure. Differential diagnoses of peripheral edema are listed in Table 82-3.
TABLE 82-3 Causes of Peripheral Edema
Collapse/Cardiac Arrest
Patients with severe heart failure of any cause are at high risk for malignant arrhythmias and thromboembolic disease such as pulmonary embolism. It is therefore not unusual for patients with severe heart failure to present with collapse or cardiac arrest. In such patients, the outlook is extremely poor. Even for patients presenting with ventricular tachycardia or ventricular fibrillation who are successfully cardioverted, the chance of surviving to discharge from hospital is low. Such patients can be considered for implantable cardioverter-defibrillators (see later and Chapter 81). Pulmonary embolism and ventricular arrhythmias are covered in Chapters 62 and 79, respectively, so will not be discussed in detail here.
 Investigations
Investigations
Electrocardiography
All patients presenting with severe heart failure require at least one electrocardiogram (ECG). In cases of diagnostic difficulty, an entirely normal ECG virtually excludes systolic heart failure as the cause of symptoms.14 In heart failure, an ECG is essential to diagnose arrhythmias such as atrial fibrillation, which may complicate management, as well as to look for evidence of myocardial ischemia or infarction and conduction abnormalities such as left bundle branch block or bradycardia due to high-degree atrioventricular block, which may respond to pacing. In patients in whom ischemia is suspected, serial ECGs are recommended, as changes may evolve during the course of the patient’s treatment. Patients with acute severe heart failure should have continuous ECG monitoring during the acute phase, as they are at high risk for malignant ventricular arrhythmias. Patients with biventricular pacemakers (discussed later) in situ may have paced QRS complexes that are narrower than in those with single-chamber right ventricular leads.
Echocardiography
Echocardiography is useful both to determine the extent of left ventricular dysfunction and to identify the cause. In cases of ischemic cardiomyopathy, regional wall motion abnormalities are commonly seen (although these can occasionally occur in cases of cardiomyopathy of other causes). Valve disease is readily identified by echocardiography. Echocardiography can be used to calculate the left ventricular ejection fraction, but in experienced hands, a qualitative assessment of left ventricular function can be equally useful. Some patients presenting with severe heart failure have preserved systolic function, and echocardiography can also be used to assess diastolic function. In the patient presenting with shortness of breath, in whom the cause is unclear, echocardiography can readily determine the presence or absence of systolic heart failure. Although a number of diagnostic criteria have been developed for assessment of the diastolic left ventricular function, their reliability in predicting intracardiac filling pressures is not always satisfactory and needs further development.15 A clear distinction can sometimes be made only by measurements of gas exchange or blood oxygen saturation or by invasive hemodynamic measurements during graded levels of exercise following the clinical stabilization.
Brain Natriuretic Peptide
Natriuretic peptides are currently emerging as a novel test in cases of heart failure. The group includes three structurally related peptides, with variable activity at three distinct natriuretic peptide receptor subtypes, of which two are of potential use in patients with heart failure. Atrial natriuretic peptide is released from the atria in response to wall stretch. Brain natriuretic peptide (BNP), so called because it was first identified in brain tissue, is mainly released by the cardiac ventricles in response to wall stretch.16 All the natriuretic peptides are elevated in acute coronary syndromes and MI, owing to release from myocytes. In addition, decompensated heart failure is associated with elevations of natriuretic peptide levels. Many possible applications for assays of these peptides have been proposed, but at present the most widely accepted indications for use of BNP (which appears to have the best sensitivity/specificity of all the natriuretic peptides) are as follows:
The use of BNP for monitoring progress in heart failure is controversial; some studies have suggested that BNP may be useful to guide treatment. Indeed, many studies have found that BNP levels may have prognostic implications. It is also important to note that BNP levels must be used in conjunction with clinical assessment of the patient, as unexpected values may occur in some patients, such as a high BNP level in a stable patient. Recent findings do not support routine use of the peptides in all dyspneic patients admitted to the emergency department.17 Of particular note is the fact that patients with severe heart failure due to cardiogenic shock may exhibit a paradoxically normal or even low BNP level. It has been suggested that myocytes in such a situation are unable to produce BNP. This theory is supported by studies of serial BNP levels in patients recovering from an episode of cardiogenic shock. An initially low BNP level is followed by a high level as recovery of myocardial function begins, and as recovery continues, the level returns to normal.
However, the measurement of BNP during admission with acute decompensated heart failure may help to assess prognosis and guide therapy following stabilization.18,19 A soluble form of ST2, an interleukin 1 (IL-1) receptor family member, has also been found to be potentially useful for identifying heart failure patients at risk of sudden cardiac death and may provide additional information to BNP.20
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree