130 Septic Shock
 Incidence
Incidence
Septic shock is the form of acute circulatory shock that occurs secondary to severe infection. The incidence of severe sepsis and septic shock is rising, partly related to medical progress that allows individuals to survive longer, resulting in increased numbers of older, debilitated, or immunocompromised patients passing through the intensive care unit (ICU). Some 10% to 15% of ICU patients develop septic shock at one time or another, and the mortality rate is 50% to 60%.1 Somewhat lower mortality rates have been reported in some trials evaluating the effects of new therapeutic interventions,2 but such studies include a number of exclusion criteria that are often associated with high mortality rates—cirrhosis, immunosuppression, and “do-not-resuscitate orders,” for example—so it is perhaps not surprising that mortality rates are lower in these therapeutic trials than in “real life.”
 Etiology of Septic Shock
Etiology of Septic Shock
The organisms involved in severe sepsis and septic shock are most often bacterial. While in the past gram-negative organisms were most commonly implicated, increasingly gram-positive organisms are isolated, such that roughly similar numbers of gram-positive and gram-negative organisms are now involved.1 Septic shock can also be caused by a fungal or parasitic infection. In a third of patients, no infectious agent is identified.1 About half of infections are nosocomial in origin. Although an infection can arise anywhere, the lung is presently the most common source of infection (40%), followed by the abdomen (20%), indwelling venous and arterial catheters and primary bacteremias (15%), and the urinary tract (10%).1
 Pathophysiology of Septic Shock
Pathophysiology of Septic Shock
The pathophysiology of septic shock is complex and covered in detail in Chapter 129 (Reinhart & Bloos). Essentially, the systemic sepsis response starts with recognition of an invading organism or its toxins. Among the bacterial factors, one of the best-known toxins is lipopolysaccharide (LPS), which is part of the outer gram-negative bacterial membrane, but other bacterial-derived factors include lipoteichoic acid and peptidoglycan. In certain cases, essentially infections involving Staphylococcus aureus or β-hemolytic group A Streptococcus, the formation of superantigens results in toxic shock syndrome.
The early humoral response involves the complement and contact (kinin-kallikrein) systems. Immune cells, principally monocytes/macrophages and polymorphonuclear neutrophils (PMN), are not only able to recognize pathogenic agents and their products so they can phagocytose and destroy them, but also release a series of mediators which can themselves activate other cells. Among the cell membrane receptors implicated in the recognition of pathogenic agents are the so-called Toll-like receptors (TLR), a family of 10 members. Of these, TLR4 is the receptor for LPS; TLR2 for a number of products from gram-positive bacteria such as peptidoglycans, mycobacteria, and yeasts; and TLR9 for bacterial DNA.3 In response to cellular stimulation, intracellular signaling is activated, resulting largely in activation of transcriptional factors, including nuclear factor kappa B (NF-κB), which in turn are responsible for initiation of proinflammatory reactions. A number of cytokines, two of the key players being tumor necrosis factor alpha (TNF-α) and interleukin (IL)-1 that interact synergistically, are released by macrophages and other cells. TNF-α and IL-1 are particularly important proinflammatory cytokines whose administration in animals can reproduce all the features of septic shock including hypotension and development of multiple organ failure. A host of secondary mediators including lipid mediators, oxygen free radicals, proteases, and arachidonic acid metabolites are also released by macrophages, PMNs, and other cells. Vasodilator substances such as nitric oxide (NO) and prostaglandins are released by endothelial cells and are responsible for the early hemodynamic changes of sepsis. NO in particular is a powerful vasodilator acting on vascular smooth muscle. Increased NO production is essentially due to induction of inducible NO synthase (iNOS) by proinflammatory cytokines. The formation of large quantities of NO can also have secondary toxic effects on cells. NO can block mitochondrial respiration, directly via inhibition of cytochrome a,a3 and by reaction with superoxide radicals, resulting in the production of peroxynitrite, which inhibits various phases of mitochondrial respiration.4 These effects result in depletion of cellular adenosine triphosphate (ATP) and potentially severe detrimental effects on cell function. It is important to note that the inflammatory response also causes release of vasoconstrictor substances including thromboxane and endothelins.
Other effects of the inflammatory reaction that accompanies septic shock include expression of adhesion molecules on vascular endothelium and circulating cells (platelets, PMNs, and monocytes), allowing adhesion of activated leukocytes and their migration into subendothelial tissues. Alterations in intercellular endothelial junctions result in increased capillary permeability and generalized edema. Alterations of coagulation and fibrinolysis complete the picture, with proinflammatory mediators creating a procoagulant state. Briefly, activation of tissue factor on the surface of various cells, particularly monocytes and endothelial cells, initiates the coagulation system.5 In addition, sepsis causes a significant reduction in plasma levels of natural anticoagulants such as protein C, protein S, and antithrombin by reducing their synthesis, increasing their consumption, and increasing their clearance. Thrombolysis is also stimulated with an increase in levels of plasminogen activator inhibitor (PAI-1). The net result is a balance in favor of procoagulant processes, often leading to disseminated intravascular coagulation (DIC) and participating in the microcirculatory disorder that leads to multiple organ failure and death in many patients with severe sepsis.
During the sepsis response, antiinflammatory mediators including IL-4 and IL-10 are also released, which limit the effects of the proinflammatory mediators and can lead to a state of relative immunosuppression, sometimes called immunoparalysis.6
 Classification
Classification
Following recommendations from the Sepsis Conference,7 patients with septic shock may be classified according to the letters PIRO:
P = Predisposing Factors
Each patient has specific characteristics. For example, an individual receiving long-term immunosuppressant therapy requires a different approach than someone who was previously healthy. Factors associated with lifestyle, such as alcoholism, may influence the course of septic shock.8 Patient age and gender may also be important. Increasingly, genetics are being considered, and studies are discovering which genetic factors can influence the development of and survival from severe sepsis. In particular, a polymorphism of the TNF-α promoter gene has been associated with increased risk of sepsis.9 Multiple other polymorphisms that may influence the response of the host to pathogenic organisms have been described, including for IL-1 receptor antagonist (IL-1ra), TLR2, and IL-6.10 Improved understanding of these aspects should help better direct therapeutic strategies.
I = Infectious Insult
This refers to the specific characteristics of the infection, that is, the agent or pathogen involved (e.g., gram-positive versus gram-negative, bacteria versus fungus),11 the source of the sepsis (e.g., urinary tract versus respiratory tract),12 and the degree of extension of the infection (e.g., pneumonia confined to one lobe of one lung versus generalized bilateral lung involvement, appendicitis versus generalized peritonitis). All these factors can influence the severity of the sepsis response and the patient’s likely response to therapy.
R = Host Response
This refers to the factors involved in the inflammatory response of the host to the infection, assessed largely by the presence or absence of the signs and symptoms of sepsis (e.g., degree of elevation of white blood cell count, CRP, procalcitonin). Each patient mounts a different response dependent on various factors including those previously discussed, and a patient’s response will vary with their clinical course and treatment.13
O = Organ Dysfunction
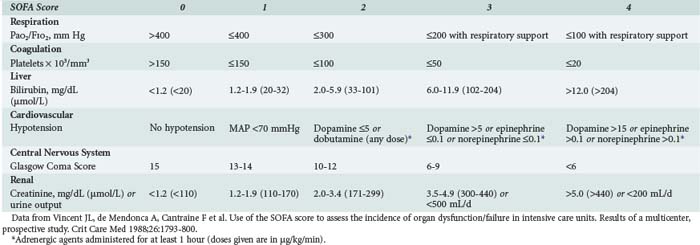
This refers to the degree of organ dysfunction related to sepsis and can be evaluated using various scoring systems, including the SOFA (sequential organ failure assessment) score,14 which uses objective, readily available measures to quantify the dysfunction of six organ systems (Table 130-1). Dysfunction of each organ is rated according to a scale (0 [normal function] to 4 [organ failure]), and individual scores can then be summed to provide a total. Individual organ function as well as a composite score can thus be followed during the course of disease and treatment.
 Clinical Presentation
Clinical Presentation
One may anticipate that patients with septic shock will have fever, hyperleukocytosis, and other typical features of sepsis, but unfortunately this is not always true. Fever may be an important clue, but moderate fever can be found in other types of shock. More importantly, fever is often absent in septic shock; in fact, hypothermia may be present in 15% to 20% of cases, and this symptom is associated with higher mortality rates.15 Hyperleukocytosis is also nonspecific and can be found in other types of circulatory failure. Likewise, lactic acidosis, a hallmark of all types of circulatory failure, is usually compensated by hyperventilation, so tachypnea is not specific for septic shock. Similarly, tachycardia can be the result of the circulatory alterations associated with any type of shock.
 Monitoring
Monitoring
Any patient with septic shock requires monitoring with an arterial catheter to enable reliable and continuous assessment of arterial pressure. Changes in systolic and pulse pressure in mechanically ventilated patients during the respiratory cycle may also indicate a greater likelihood of response to a fluid challenge.16 The arterial catheter also facilitates blood sampling, notably for blood gas analysis.
Invasive Versus Less-Invasive Monitoring
The role of the pulmonary artery catheter (PAC) in critically ill patients has been questioned.17 However, although no study has conclusively demonstrated positive effects of this type of monitoring on outcome,18–20 information obtained from the PAC may help in guiding patient management.21 The PAC is useful not only for monitoring pulmonary artery occlusion pressure (PAOP) and cardiac output but also allows assessment of mixed venous oxygen saturation (SvO2), a highly useful parameter because a fall in SvO2 is generally associated with inadequate oxygen transport. Importantly, the PAC is not necessary in all patients but is likely to be of use in complex cases, particularly in patients with concomitant cardiopulmonary disease.
Less-invasive monitoring techniques are increasingly being used. Echocardiography can provide useful additional information, largely to visualize the degree of ventricular filling and ejection volume. However, echocardiography requires an experienced operator, gives no information on the adequacy of cardiac output for the patient’s needs, and is difficult to perform continuously, so information is intermittent. Other less-invasive methods of monitoring cardiac output include PiCCO, LidCO, transesophageal Doppler techniques, and even bioimpedance or bioreactance techniques.22 However, measurement of cardiac output in isolation is not very helpful in most critically ill patients.
Blood Lactate Levels
Blood lactate level is an important biological variable in determining the adequacy of perfusion and oxygenation. Normal blood lactate level is around 1 mEq/L, and hyperlactatemia becomes pathological above 2 mEq/L. Although in other forms of circulatory shock, hyperlactatemia is due to cellular hypoxia, in septic shock additional mechanisms may play an important role in raising blood lactate levels. In sepsis, blood lactate levels may be raised by an increase in cellular metabolism, by inhibition of pyruvate dehydrogenase, and by reduced clearance. Repeated measurements enable one to assess the efficacy of treatment and have a predictive value superior to derived oxygenation parameters.23 The evolution of blood lactate levels enables a global evaluation of the state of the shock, although in view of the relatively slow rate of change, blood lactate levels cannot be used to guide resuscitation.
Peripheral Perfusion Parameters
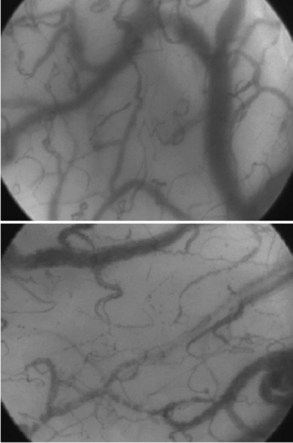
Other techniques for monitoring peripheral perfusion have been developed. While the sublingual region is not one that would immediately seem to be of most interest, it is easily accessible, and using techniques of orthogonal polarization spectral (OPS) or sidestream darkfield (SDF) imaging, heterogeneity of microcirculatory flow and reduced perfused vessel density and proportion of perfused vessels can be observed (Figure 130-1) and quantified in patients with sepsis.24,25 Moreover, the impact of therapeutic interventions on such changes can be monitored,26,27 opening the possibility that monitoring the microcirculation could be used to guide treatment.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree