Fig. 18.1
The target level of sedation is different from the first period, devoted to clinical stabilization and protective ventilation, to the second one, when patients need to receive lighter sedation, intended to join and maintain a calm, conscious, and cooperative neurological state
18.2 The Key Importance of Continuous Neurological Assessment with Validated Tools
International guidelines clearly recommend systematic assessment of pain, agitation/sedation, and delirium in all ICU patients, with validated tools [4–6]. They provide repeatable and comparable measurements, able to adequately titrate the analgesic, sedative, and antipsychotic therapy. The goal is to control the stress response and the neurological symptoms together keeping ARDS patients awake, cooperative, and well adapted to the necessary invasive procedures as soon as possible [28]. An excessive use of neuroactive drugs [26] correlates with longer mechanical ventilation and ICU stay, as well as with an increased risk of serious neurological consequences in both the short- and long-term [10, 29].
Among the validated tools, the scales with the highest psychometric properties [30] are the Verbal Numeric Rating (VNR), the Behavioral Pain Scale (BPS) [31], or the Critical-Care Pain Observation Tool (CPOT) [32] for the pain assessment, the Richmond Agitation-Sedation Scale (RASS) [33] and the Sedation-Agitation Scale (SAS) [34] for the agitation/sedation assessment, and the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) [35] and the Intensive Care Delirium Screening Checklist (ICDSC) [36] for the delirium assessment.
Pain assessment has become culturally essential for intensivists, both nurses and physicians. Proper identification of pain symptoms in critically ill patients represents a challenge because of complexity in communication with patients needing endotracheal respiratory prostheses, because of altered state of consciousness or because of neuroactive drugs use. To address these issues, specific behavioral scales were designed and validated both in unconscious/sedated [37] and in conscious/awake patients [38] and established as a valid, reliable, and simple tool to be used in clinical practice.
Regarding sedative drugs, their under- or overuse may compromise clinical stability. Life-threatening side effects of untreated agitation and stress response are evident: self-removal of life-sustaining devices, tachypnea, tachycardia, hypertension, sustained hypoxemia, and hypercarbia due to uncoordinated mechanical ventilation [6]. At the same time, deeper-than-necessary sedation increases the length of ICU stay [5], the duration of mechanical ventilation [21], the sepsis severity [8], and the onset of new neurological failures both during hospitalization like delirium and after discharge [29] like the development of psychological reactions of traumatic nature (posttraumatic stress disorder, PTSD). In order to obtain the best sedative titration, the use of validated tools is mandatory. For example, the RASS describes ten levels of sedation/agitation through observation and verbal and physical stimulation. Scores range from −5 (unconscious, unresponsive to voice and physical stimuli) to + 4 (overtly combative, violent, immediate danger to staff), adequately describing the possible neurological condition needing an immediate intervention.
While regarding pain and agitation evaluation encouraging results are present in literature [39], delirium recognition [40] and assessment is more challenging, since its success is linked to an effective and lasting staff training [41]. Delirium has a very high prevalence in critically ill patients [42], with a direct relationship between increased morbidity and mortality and the duration (in days) of delirium [43, 44]. The presence and duration of delirium also correlates with a significant deterioration in the quality of life after ICU discharge [45]. Large literature recommends the use of validated tools [46], like the Confusion Assessment Method for the ICU (CAM-ICU) and the Intensive Care Delirium Screening Checklist (ICDSC). The CAM-ICU has excellent sensitivity and specificity and is based on only four items (acute modification of consciousness, inattention, disorganized thinking, altered level of consciousness). This allows a shorter training to teach ICU staff members a validated way to evaluate delirium.
All these measurements have to be reported in the clinical chart at least once per shift, together with an evaluation of adequacy of sedative treatment. The neurological monitoring plays a key role in ARDS patients, and it is quick to perform. A complete example of evaluations recommended by international guidelines is reported in Fig. 18.2. Physicians have to state the desired sedation target for that specific patient at that specific moment of clinical course; nurses, on their part, report the actual neurological state, describing pain, anxiety, agitation, sleep, need for physical restraints, and delirium, together with a comprehensive evaluation of sedative therapy, in order to get the most adequate treatment titration.
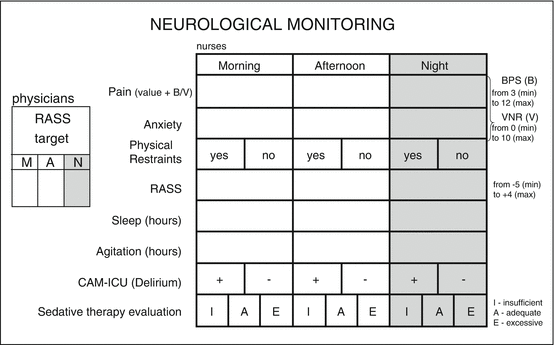

Fig. 18.2
An example of very simple neurological monitoring card, used in a general ICU of the University Hospital (A.O. San Paolo, Milano), to be added within a dedicated spot in nursing sheets. For each shift – morning, afternoon, and night – physicians have to state the target level of sedation, while nurses have to monitor and record, through appropriate validated tools, neurological parameters, and also to evaluate the adequacy of sedative therapy as prescribed by physicians
18.3 Sedation Assessment
The appropriate target level of sedation primarily depends on a patient’s acute disease process and on the therapeutic and supportive interventions required. After the first few days of ICU stay, characterized by clinical stabilization and invasive procedures, the sedation target is a calm patient, awake during the day and asleep at night. The use of deep levels of sedation to facilitate mechanical ventilation or painful procedures should be minimized with ventilation setting optimization and adequate analgesia, rather than deepening unconsciousness [12, 47].
The appropriate balance of sedation and analgesia is difficult to be achieved and maintained. Without a rational agreement upon “target levels” of sedation, different members of the healthcare team will have disparate treatment goals, increasing the chance for iatrogenic complications and potentially delaying recovery [48].
The target level of sedation should be discussed and defined at the beginning of each staff shift and reevaluated regularly as the clinical condition of the patient changes. The pharmacological treatment should be planned with the appropriate flexibility to allow titration to the desired endpoint, anticipating fluctuations in sedation requirements throughout the day. Frequent monitoring with validated tools improves communication among clinicians and plays an important role in detecting and treating pain, agitation, and delirium while avoiding excessive or prolonged sedation [25].
18.3.1 Sedation Assessment with Objective Methods
Within several objective methods to sedation assessment proposed, none of them have fully yielded satisfying results. For example, bispectral index (BIS) monitor is a four-channel electroencephalographic (EEG) monitor which generates a single number that correlates with depth of consciousness during general anesthesia. The poor correlation between BIS and validated ICU sedation scales is related with BIS values variability at the awake/agitated levels and of the electromyography (EMG) interference [49]. Based on the analysis of EEG signal irregularity, the entropy monitor also utilizes the EMG signal, which may provide information useful for assessing whether a patient is responding to an external, painful stimulus, but without adding useful information to sedation assessment. In this context, some advantages could be offered by the responsiveness index [50]. Auditory evoked potentials (AEP) are electrophysiological responses of the nervous system to standard sensory stimulation transmitted through headphones. These methods may have a role in monitoring sedation levels only in patients needing deep sedation, or receiving neuromuscular blocking agents, as in this circumstance sedation scales cannot be used [49].
Monitoring blood drug values is useful only when there is a correlation between plasmatic concentration and pharmacological effect [51]. However, ARDS patients may be affected by renal and hepatic dysfunctions that impair their ability to metabolize and excrete drugs. Moreover, hypoxia, inflammatory mediators, and abnormal diets are common, too, and all affect enzymatic function. Thus, this method cannot be recommended for sedation monitoring [52, 53]. Spontaneous, non-propulsive lower esophageal contractility (LOC) is definitely stress related and increases in frequency as the dose of anesthetic is reduced. Deepening of anesthesia resulted in progressive suppression of LOC. However, LOC has great inter-variability and is affected by drugs such as atropine. The electromyogram responsiveness is not sufficiently sensitive to monitor sedation in ICU patients [51].
Actigraphy provides a continuous measure of body movements and was initially developed to measure sleep-wake cycles. This small electronic device containing an accelerometer continuously senses and records minimal movements, summarizing such data in numerical form. Wrist actigraphy provides useful nonspecific observations in ICU patients. Even if it does not discriminate the lack (or the excess) of analgesics and sedatives from other acute neurological dysfunctions, preliminary observations suggest that the measurement of body movements could provide a timely indication of acute changes in neurological status generating motor agitation or hypoactive behavior [54]. This objective method is relatively new in this context. It presents interesting properties, worthy of future investigation [55].
18.3.2 Sedation Assessment with Subjective Methods
Individual assessments of sedation, performed at the bedside by nurses or physicians, can be hampered by a lack of objectivity. Guidelines recommend establishing a sedation target and regularly redefining it for each patient, using a validated sedation assessment scale. The use of such a scale is a key component of sedation algorithms [1, 4–6]. It helps in managing agitation and establishing a target level of sedation for medication titration, in order to promptly detect oversedation when the target level is exceeded. All sedation algorithms recommend to use a sedation scale, such as Ramsay Sedation Scale (RSS), Richmond Agitation-Sedation Scale (RASS), and Riker Sedation-Agitation Scale (SAS) [56].
The use of a scale to assess level of consciousness dates to the introduction of a six-point scale by Ramsay et al. (RSS) more than 40 years ago [57]. Nowadays, it continues to be a widely used scale for monitoring sedation in daily practice [58]. Some experts consider that it is more a scale of consciousness than a tool for the measurement of sedation. RSS has been extensively tested but it has never been validated. Moreover, it does not grade agitation. Consequently, this scale is excessively subjective and has poor validity. Many other scales have been proposed [59]; some of them are not validated. They are not recommended for clinical use.
The ideal scoring system should be easy, reliable, sensitive, and with minimal interobserver variability. Moreover, it should give no or minimal additional discomfort to the patient. Even though a complex scoring system is not suitable for the ICU, oversimplification brings risk of neglecting important information. Most of the proposed tools are a compromise between accuracy and time required for evaluation of sedation [60].
Recently developed scales often combine the sedation/arousal domain with an assessment of agitation, like the SAS, the RASS, the Motor Activity Assessment Scale (MAAS), the Observer’s Assessment of Alertness and Sedation (OAAS), the Nursing Instrument for the Communication of Sedation (NICS) [61], and the Bloomsbury Sedation Score (Fig. 18.3).
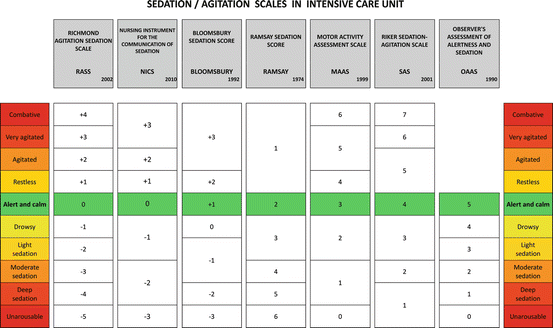

Fig. 18.3
Among the different validated tools for agitation and sedation assessment, each ICU should collegially choose the one to use
Unlike other validated instruments, the RASS separates verbal from physical stimulation so that the patient’s level of arousal may be graded according to the potency of the stimulus. Interestingly, RASS is validated also to assess patients’ sedation over time, both in spontaneously breathing/mechanically ventilated and in sedated/nonsedated critically ill patients.
Other multiple-item sedation scales are described in literature. The Adaptation to the Intensive Care Environment scale (ATICE) consists of five items [62]: awareness and comprehension combined in a “Conscious” domain; calmness, ventilator synchrony, and facial relaxation are combined in a “Tolerance” domain. As for ATICE, the Minnesota Sedation Assessment Tool (MSAT) evaluates the level of consciousness of patients receiving invasive mechanical ventilation. It measures arousability, spontaneous muscle activity, and global sedation quality. The Vancouver Interaction and Calmness Scale (VICS) consists of two five-item subscales quantifying separately calmness and interaction with operators. These more complex scoring systems are usually adopted in clinical trials to evaluate a new drug or a new objective tool for sedation assessment, whereas in daily practice, easily applied scores are usually preferred.
18.3.3 Choosing and Implementing an Evaluation Scale
Desirable features of sedation evaluation instruments should include rigorous multidisciplinary development; ease of administration, recall, and interpretation; well-designed discrete criteria for each level; sufficient sedation levels for effective drug titration; assessment of agitation; and demonstration of inter-rater reliability and validity in relevant patient populations [48]. Each ICU has to choose the best tools for its patient population and to plan specific intervention to introduce it in daily care [63].
Teaching protocols used for implementation of sedation scales have shown good results among ICU caregivers. Different methods have been used to implement evaluation tools in clinical practice. Typically, they are based on introductory in-service for nurses and operators followed by graded, staged educational interventions at regular intervals. Web-based, freely available teaching interventions have been also proposed (www.icudelirium.org, www.sedaicu.it).
Some emerging problems remain, particularly about the fluctuation of consciousness. ARDS patients are prone to sudden changes in their state of consciousness due to the effects of drugs, sleep disruption, organic and metabolic disease, or delirium. Assessment of sedation once a shift is indispensable but not sufficient. Among the different possibilities (minimal/maximal level, prevalent level, worst level), it is important to state the duration of each value within the observed shift. Sedation and agitation need to be reassessed both on a regular basis and during any clinical modification, to promptly capture all the modifications requiring intervention. Moreover, it is relatively common for patients to manifest sudden aggressive behavior when recovering from sedation and without fully awakening. For this reason, it is important to boost interdisciplinary communication between nurses and physicians in order to be aware of and prevent these problems.
Lastly, making a sedation assessment during the night is frequently challenging. Most analgesics and sedatives are known to make patients sleepy, but without reaching a restorative, physiological sleep [64]. If a critically ill patient appears calm and keeps his/her eyes closed during the night, he/she should not be stimulated just to make a sedation assessment. He/she could be observed during unavoidable procedures happening in the ICU during the night, in order to discriminate normal sleep (with arousals due to noise or light) from sedation or coma.
18.4 Clinical Practice Flowcharts for Pain, Agitation, and Delirium Management
Recognition that heavy sedation may increase mortality and morbidity has led to a new approach that maximizes the comfort of the patients while they remain awake, interactive, and oriented. This new approach relies on strategies such as daily interruptions of sedation, analgesia-based sedation, enteral sedation, avoidance of paralytic agents, early mobilization, and use of validated tools for sedation assessment [1]. In recent years, many guidelines have been proposed [4–6], representing a guide to symptom-oriented prevention, diagnosis, and treatment of delirium, anxiety, stress, and protocol-based analgesia, sedation, and sleep management. They comprehensively describe all attentions to be paid to perform the best neurological treatment. Nevertheless, even in guidelines with a high-quality rating, numerous recommendations have moderate or low levels of evidence [65].
Overall, the principal recommendation regarding analgesia is to evaluate pain and maintain its level ≤ 4/10, by beginning early than late the treatment of pain, and by using opioid drugs first, together with non-opioid and multimodal analgesia techniques. A simple and practical flowchart at the bedside is presented in Fig. 18.4.
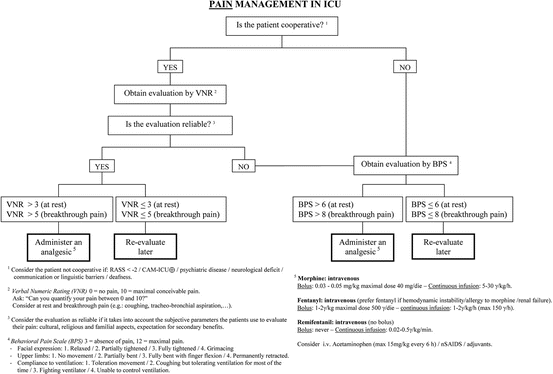

Fig. 18.4
This flowchart can be used at the bedside, to adequately manage pain in critically ill patients
Regarding sedation, a target RASS of 0/−1 is recommended for all ICU patients, with the use of deep sedation reserved only for patients with specific indications (e.g., early ARDS patients, requiring neuromuscular blocking or prone positioning). In particular, intensivists should consider the specific indication and individual goal of sedation and the pharmacokinetics/pharmacodynamics of each drug used. Non-benzodiazepine drugs, such as propofol or dexmedetomidine, have to be preferred. The need for sedation varies widely among different patients and with the illness course, and it has to be defined among “deep sedation”, “cooperative/awake sedation,” and “no sedation,” always preferring superficial levels of sedation and promoting early mobilization. The use of containment measures in episodes of severe agitation has to be performed in this order: first verbal, then pharmacological, and finally physical, considering that neuroactive drugs should not be administered in excess as a form of “chemical immobilization.” In the sedation flowchart (Fig. 18.5), it is clear that defining a target and evaluating the actual level of sedation or agitation with validated tools are absolute priorities. The choice of the specific sedative drug, albeit important, comes only after a clinical reasoning focused on managing the organic/metabolic causes and the problems that may be caused by adjustable invasive devices.
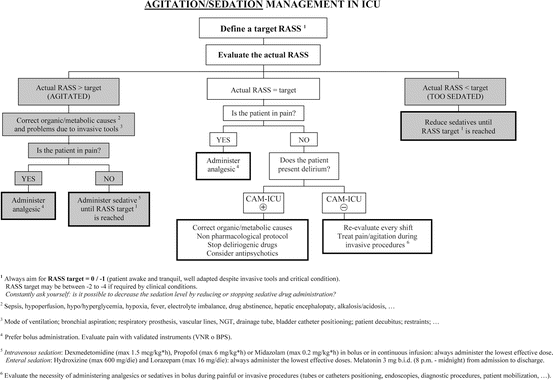

Fig. 18.5
This flowchart can be used at the bedside, to adequately manage agitation symptoms and to titrate sedative therapy for critically ill patients
Recommendations about delirium are prescribed first to identify modifiable risk factors (Fig. 18.6) and to detect regularly the appearance of delirium by using the CAM-ICU. In case of delirium appearance, it is indicated to use a non-pharmacological protocol first, along with the suspension or decreasing of deliriogenic therapies, and then to select the most appropriate drug (haloperidol, atypical antipsychotics, or dexmedetomidine; avoid the use of benzodiazepines) and titer its dose to the lowest effective dose.
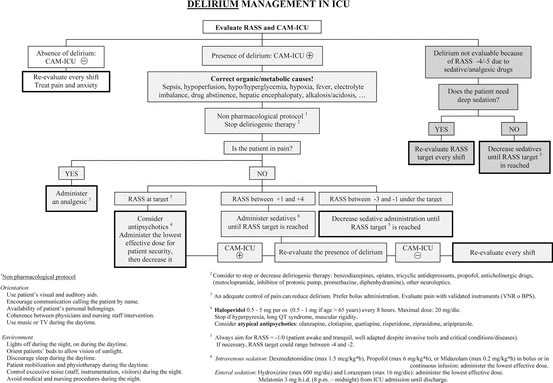

Fig. 18.6
This flowchart can be used at the bedside, to timely manage delirium in ICU
18.5 Sedation Protocols Presented in Literature
Beyond the choice of the specific drug [66–68], the most frequently used method for administering sedatives is the continuous intravenous route, because of its pharmacokinetic properties [63]. Intravenous infusions present predictable and easy to handle onset/offset properties, justifying the search for an early goal-directed sedation strategy [69]. Although these characteristics are necessary in short ICU stays, they may be useless, or even dangerous, for patients requiring more than 3 days of mechanical ventilation [27]. When using potent drugs, it is easy to incur in overadministration albeit goals are established and adequate [25]. Moreover, daily awakening trials produce far-from-physiological neurological fluctuations [70], and continuous deep sedation does not permit patients to recall factual memories, which has been proven effective in preventing PTSD [71].
If continuous intravenous infusion is used, the daily interruption of sedatives and analgesics is recommended in order to reduce the total administered dosage and to perform a spontaneous breathing trial, if allowed by the respiratory condition of the patient. The purpose is to reduce the development of complications and the duration of mechanical ventilation [17]. This strategy may prove less effective if specific and ICU team-shared protocols are used [72].
Many quite different sedation protocols have been presented in literature [10, 73, 74]. Some of them essentially rely on the use of different drug doses [75], with the aim to different sedation levels [56, 76]. Other protocols are based on nursing-implemented algorithms [13, 77–79] or on analgesia-first sedation [21, 80]. Moreover, tested ways to optimize sedation management in ICU are patient-controlled sedation [81] or automated sedation in patients needing deep sedation [82], the use of inhaled halogenates [83, 84], or the enteral administration of drugs [28, 85]. All these protocols rely on the continuous and adequate neurological assessment made with validated tools, in order to measure not only pain, agitation, and delirium but also level of consciousness and patient mobilization.
The most promising protocol, in terms of efficacy, recommends to join the sedation strategies with early physiotherapy [86], mobilization [87], and occupational therapy [88, 89], also engaging patient families. Even if implementing such protocols is not simple [90], from the first presentation made in 2010 [91, 92], it has offered the best results in terms of effectiveness [93]. Briefly, following the acronym ABCDEF, the authors made these suggestions:
Assess, prevent, and manage pain.
Both spontaneous awakening trial and spontaneous breathing trial.
Choice of analgesia and sedation.
Delirium: assess, prevent, and manage.
Early mobility and exercise (goal-directed early mobilization).
Family engagement and empowerment.
18.6 Special Circumstances
Regarding ARDS patients needing extracorporeal life support systems (ECLS), there is a gray area about sedation, where safety aspects and the ability to positively influence recovery must be balanced. Patients on ECLS have numerous risk factors to develop both delirium during the ICU stay and PTSD after discharge. Hyperactive delirium or agitation can be life-threatening for these patients, so that a consequent monitoring and a symptomatic therapy of stress, anxiety, delirium, pain, and insomnia is essential to safely achieve a target RASS of 0. The higher level of alertness allows the patient to actively partake in physical exercises [94] that is considered a feasible and safe goal [95, 96]. International guidelines recommend a strict definition of sedation targets for patients on ECLS, including frequent clinical monitoring and continuous adjustment of the level of sedation required [4].
Positioning therapy has been demonstrated effective in ameliorating prognosis of severe ARDS patients. It is used for prophylaxis and treatment of respiratory dysfunctions and requires an individual sedation target. Changes of the position frequently represent a challenge for the symptomatic treatment of anxiety, stress, and pain. Therefore, a symptom-orientated therapy should be adapted for changing demands during positioning therapy. Though a deep sedation may be indicated for patient repositioning [97], an excessive sedation should be avoided through the use of objective tools based on EEG analysis, as previously described.
The use of non-depolarizing neuromuscular blockers in patients with severe ARDS is suggested during the first 48 ICU hours. Despite this practice, it is suggested not to routinely increase the dose of sedatives, when accompanied by an infusion of opiates, even in patients subjected to permissive hypercapnia [98]. To control respiratory rate, fentanyl is recommended as the analgesic of choice in patients with hemodynamic instability, bronchial asthma, or COPD, with respect to the other opiates. Methadone via the enteral route could be used in patients receiving opiates for more than 5 days, but still needing mechanical ventilation. Once tracheostomy is performed, it is advisable to consider a decrease in sedative and analgesic regimens.
After the hyperacute phase of respiratory failure, a defined sedation and analgesia monitoring and dose adjustment protocol is recommended, to shorten the weaning process. This protocol should include daily evaluation of sedation, an awakening test and a spontaneous breathing test. It is advisable not to use benzodiazepines in the withdrawal of MV. Dexmedetomidine is recommended in case of weaning difficulties, in patients with withdrawal syndrome, or after failed attempts of weaning secondary to agitation and delirium [67]. Low-dose remifentanil in continuous infusion is another effective alternative during weaning process. Music therapy is a possible non-pharmacological adjuvant to sedation [99]. Melatonin supplementation could be useful to decrease the need for sedative drugs, then shortening the ventilation length [100], and to restore the sleep-wake rhythm.
References
1.
Sessler CN, Varney K (2008) Patient-focused sedation and analgesia in the ICU. Chest 133(2):552–565. doi:10.1378/chest.07-2026 CrossRefPubMed
2.
Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, Moons KG, Geevarghese SK, Canonico A, Hopkins RO, Bernard GR, Dittus RS, Ely EW (2013) Long-term cognitive impairment after critical illness. N Engl J Med 369(14):1306–1316. doi:10.1056/NEJMoa1301372 CrossRefPubMedPubMedCentral
3.
Fraser GL, Riker RR (2007) Sedation and analgesia in the critically ill adult. Curr Opin Anaesthesiol 20(2):119–123. doi:10.1097/ACO.0b013e32808255b4 CrossRefPubMed
4.
Baron R, Binder A, Biniek R, Braune S, Buerkle H, Dall P, Demirakca S, Eckardt R, Eggers V, Eichler I, Fietze I, Freys S, Frund A, Garten L, Gohrbandt B, Harth I, Hartl W, Heppner HJ, Horter J, Huth R, Janssens U, Jungk C, Kaeuper KM, Kessler P, Kleinschmidt S, Kochanek M, Kumpf M, Meiser A, Mueller A, Orth M, Putensen C, Roth B, Schaefer M, Schaefers R, Schellongowski P, Schindler M, Schmitt R, Scholz J, Schroeder S, Schwarzmann G, Spies C, Stingele R, Tonner P, Trieschmann U, Tryba M, Wappler F, Waydhas C, Weiss B, Weisshaar G (2015) Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS-Guideline 2015) – short version. Ger Med Sci GMS E-Journal 13:Doc19. doi:10.3205/000223 PubMed
5.
Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, Coursin DB, Herr DL, Tung A, Robinson BR, Fontaine DK, Ramsay MA, Riker RR, Sessler CN, Pun B, Skrobik Y, Jaeschke R (2013) Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 41(1):263–306. doi:10.1097/CCM.0b013e3182783b72 CrossRefPubMed
6.
Celis-Rodriguez E, Birchenall C, de la Cal MA, Castorena Arellano G, Hernandez A, Ceraso D, Diaz Cortes JC, Duenas Castell C, Jimenez EJ, Meza JC, Munoz Martinez T, Sosa Garcia JO, Pacheco Tovar C, Palizas F, Pardo Oviedo JM, Pinilla DI, Raffan-Sanabria F, Raimondi N, Righy Shinotsuka C, Suarez M, Ugarte S, Rubiano S (2013) Clinical practice guidelines for evidence-based management of sedoanalgesia in critically ill adult patients. Med Intensiva/Sociedad Espanola de Medicina Intensiva y Unidades Coronarias 37(8):519–574. doi:10.1016/j.medin.2013.04.001 CrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





