144 Rickettsial Diseases
Proteobacteria are small gram-negative obligate intracellular organisms that can be divided into two classes: Alphaproteobacteria including Rickettsiaceae (genus Rickettsia) and Anaplasmataceae (with four genera: Ehrlichia, Anaplasma, Neorickettsia, and Wolbachia), and Gammaproteobacteria that include Coxiellaceae (genus Coxiella).1
The Rickettsia genus is divided into the spotted fever group (SFG), which comprises about 15 different species of human pathogens, the typhus group, and the scrub typhus group.2 The spotted fever group includes arthropod-borne diseases and comprises mainly Rickettsia rickettsii, the agent of Rocky Mountain spotted fever, and Rickettsia conorii, the agent of Mediterranean spotted fever.2 The typhus group comprises Rickettsia prowazekii, causing louse-borne epidemic typhus, and Rickettsia typhi, the agent of the fleaborne murine typhus. The scrub typhus group includes Orientia tsutsugamushi, a mite-borne disease.2 Part of the clinical manifestations and sequelae associated with most human rickettsioses are due to the bacteria’s affinity for the blood vessels’ endothelium, leading to damage to the vascular endothelium, triggering vascular inflammation, and compromising vascular permeability.3,4 Exceptions are Rickettsia akari and O. tsutsugamushi, which invade and multiply in the monocytic cells.4
Ehrlichioses are zoonoses increasingly recognized as human pathogens. The human pathogens of this family, depending on the causative species, invade various target cells of the hematopoietic and lymphoreticular systems.4 Coxiella burnetii, the agent of Q fever, infects many mammal species, including humans. The clinical presentation and evolution of Q fever seem to be related to host immune response, especially to tumor necrosis factor (TNF)-α and IL-10 production by stimulated monocytes.4,5 The ability of macrophages to kill the organisms and the clinical presentation of infection seem to depend on the immune status of the patient.4
 Rickettsial Diseases
Rickettsial Diseases
Spotted Fever Group
Rickettsia rickettsii is the causative agent of Rocky Mountain spotted fever (RMSF), an arthropod-borne disease, transmitted by Dermacentor ticks.2,6,7 RMSF occurs mainly in rural and suburban locations throughout North America, Central America, and parts of South America (Colombia, Bolivia, Brazil).8 The disease is highly seasonal, with the highest incidence during late spring and summer months.8 After a 2- to 14-day incubation period, patients typically develop fever, myalgia, and severe headaches.4 The inoculation eschar is rarely found. The major diagnostic sign, the petechial rash (Figure 144-1), usually appears 3 to 5 days after the onset of fever, although older patients and black patients might not develop the rash.4,9 The rash is usually first noted around the wrists and ankles, and then involves the palms and soles, with centripetal progression.4,8,9 Because R. rickettsii produces small-vessel injury, patients can present with other symptoms such as seizures, focal neurologic deficits, transient deafness, meningoencephalitis, gastrointestinal symptoms (abdominal pain and tenderness mimicking acute abdomen), myocarditis, pericarditis, and pneumonia.4,8 Despite widespread endothelial damage and microangiopathic thrombosis in some cases, fulminant disseminated intravascular coagulopathy (DIC) is rarely seen.8 However, fulminant RMSF has been associated with older age, black males with glucose-6-phosphate dehydrogenase (G6PD) deficiency, and possibly with alcoholism.4
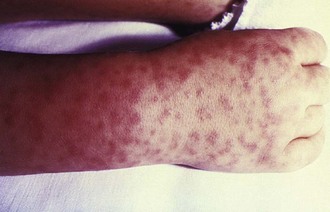
Figure 144-1 Child’s right hand and wrist displaying the characteristic spotted rash of Rocky Mountain spotted fever.
(Courtesy Public Health Image Library, Centers for Disease Control and Prevention.)
Rickettsia conorii is the causative agent of several infections designated by geographic names and differentiated by serologic techniques: Marseilles fever, Mediterranean spotted fever (boutonneuse fever), Kenya tick typhus, Israeli tick typhus, Astrakhan spotted fever, and Indian tick typhus.4,10 Several serotypes merit mention:
Rickettsia akari, the etiologic agent of rickettsialpox or smallpox rickettsia, is transmitted by Allodermanyssus sanguineus.2 One week after a mite bite, a vesicle appears, then dries and leaves a black eschar (Figure 144-2). The typical presentation of a patient with rickettsialpox is fever, papular or vesicular rash, and eschar.12 The rash does not involve the palms and soles; tender lymphadenopathy is commonly found on physical examination.12,13
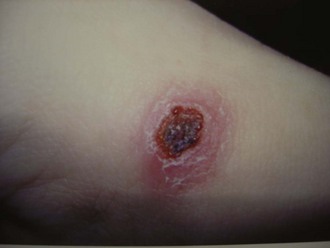
Figure 144-2 Rickettsialpox early lesion.
(Courtesy Dr. Daniel Caplivski, Division of Infectious Diseases, Mount Sinai School of Medicine.)
Rickettsia africae, transmitted in sub-Saharan Africa and West Indies by Amblyomma ticks, causes African tick bite fever.14 The disease is described mainly in people who hunted or traveled in a bushy area in southern Africa.14 In a high proportion of cases, several inoculation eschars (Figure 144-3) can be seen; patients often have lymphadenitis in the regions that drain the eschars.14 One week after the tick bite, 46% of patients develop fever, headache, myalgia, and a rash, which can be vesicular.14 The evolution is much milder than Mediterranean spotted fever.14
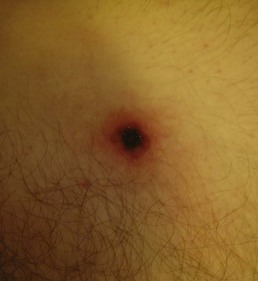
Figure 144-3 Eschar (tache noir) in a patient with Rickettsia africae.
(Courtesy Dr. Daniel Caplivski, Division of Infectious Diseases, Mount Sinai School of Medicine.)
Rickettsia parkeri infection, transmitted by Amblyomma maculatum, has been recently documented in the eastern coastal states of the United States.15 Patients present approximately 1 week after a tick bite with fever, myalgia, malaise, headache, and a maculopapular eruption that may involve the palms or soles; an eschar can be found in the majority of cases.15 R. parkeri infection seems to be a milder illness than RMSF.15
Rickettsia slovaca causes tickborne lymphadenopathy, also known as TIBOLA, a disease common in Europe and transmitted by Dermacentor marginatus ticks during winter and early spring.2,6,16–18 This disease is more prevalent in children and women; it is characterized by the presence of an eschar in the scalp and enlarged, tender, draining lymph nodes.16,18 Fever and rash are rarely observed. Postinfectious asthenia and residual alopecia at the site of the tick bite have been reported.16,18
Rickettsia helvetica has been isolated from Ixodes ricinus ticks in many European and Asian countries.19,20 The disease can be mild or self-limited with associated fever, headache, and myalgia, or it can have a more severe clinical presentation.17,19–21 Cases of fever without rash or eschar have also been reported.19
Rickettsia aeschlimannii has been isolated from Hyalomma marginatum in Africa, Corsica, and Spain and is responsible for a disease similar to Mediterranean spotted fever.22
Rickettsia australis, the etiologic agent of Queensland tick typhus, is transmitted by Ixodes holocyclus in Australia.2,23 Patients present with a rash (which can be vesicular), an inoculation eschar, and regional lymphadenopathy.23 The evolution is mild.23
Rickettsia honei causes Flinders Island spotted fever, found in continental eastern Australia and probably in Thailand.2,6,24 It is a febrile illness associated with an erythematous rash and headache; an eschar is found in 25% of patients, whereas regional adenopathy occurs in 55% of patients.2,6 R. honei subsp. marmionii, or R. marmionii, has more recently been described to cause an acute febrile illness associated with headache, myalgia, arthralgia, cough, maculopapular or petechial rash, pharyngitis, eschar, and adjacent lymphadenopathy.25,26 It has also been associated with chronic illness, but it is unclear whether the bacteria are responsible for any of the chronic symptoms or whether they are just a marker of increased immunosuppression.26
Rickettsia japonica causes Japanese or Oriental spotted fever, and it is transmitted by Haemaphysalis longicornis and Dermacentor taiwanensis in Japan and in eastern China.27–29 Patients present with fever, headache, inoculation eschar, adjacent adenopathy, and a maculopapular rash.2,6,30 Meningoencephalitis and even fulminant cases complicated by DIC and death have been reported.27–31
Rickettsia sibirica, the agent of Siberian tick typhus, is transmitted by Dermacentor marginatus and Haemaphysalis concinna.4,32 The disease has been described in Siberia and China.4 After 1 week of incubation, an ulcerated necrotic lesion appears at the inoculation site, often accompanied by regional lymphadenopathy.
Rickettsia mongolotimonae, related to R. sibirica, is transmitted by the Hyalomma asiaticum tick in Mongolia, sub-Saharan Africa, and southern Europe.33–35 The main clinical manifestation is lymphangitis associated with an inoculation eschar and satellite lymphadenopathy.33–35 Patients present with fever, severe headache, and a discrete rash.33–36
Rickettsia felis causes fleaborne spotted fever and is transmitted by the cat flea, Ctenocephalides felis; it has been documented worldwide.4,21 The disease is characterized by fever, maculopapular rash, and headache.37–39 Eschar and gastrointestinal and neurologic signs are not common.39
Typhus Group
Rickettsia typhi, the agent of murine typhus or endemic typhus, is transmitted through scratching contaminated pruritic lesions after rat flea bites.2,40 Murine typhus has been diagnosed worldwide and is prevalent in tropical and subtropical seaboard regions.41 After 1 to 2 weeks’ incubation, the disease abruptly begins with fever, nausea, myalgias, arthralgias, and headache.42–45 Gastrointestinal symptoms and a maculopapular rash that starts on the trunk, spreads peripherally, and spares the palms and soles develop later in the course of the disease.42,45 A third of patients can develop respiratory symptoms.4 Neurologic symptoms can be present, ranging from confusion and stupor to seizures and coma in severe forms.4 Severe forms of disease are described in males of African descent with G6PD deficiency and in the elderly, especially when the diagnosis is delayed; such patients can present with central nervous system abnormalities, pulmonary compromise, as well as hepatic and renal dysfunction.42,45 Splenic rupture has been reported with acute infection, patients presenting with acute abdomen.46–48 Prognosis is usually favorable, with a low fatality rate.45
Rickettsia prowazekii, the etiologic agent of epidemic typhus or exanthematic typhus, is transmitted by the human body louse, Pediculus humanus corporis (Figure 144-4).2,40,49 The human body louse lives in clothes and multiplies rapidly when cold weather and lack of hygiene allow (during war, in poor countries, and in the homeless population in developed countries).40,49 This bacterium is a potential warfare agent and has been classified in category B of biological agents by the Centers for Disease Control and Prevention (CDC).50 The disease begins abruptly with fever and headache.51 The presence of myalgia, arthralgia, and constitutional symptoms are variable.51 In more than one-third of patients, the rash can be macular, petechial, and even purpuric; the lesions are distributed mostly on the trunk and may spread centrifugally to involve the extremities; rarely, lesions are found on the soft palate and conjunctiva, but not on the face, palms, and soles; eschars are absent.51 Neurologic involvement such as delirium, stupor, confusion, and even coma is common.51 Brill-Zinsser disease, a milder form of typhus, is diagnosed during the convalescent period if the bacteria is not completely eradicated and infection persists subclinically.51,52 It is frequently underdiagnosed because the rash as well as a history of recent exposure can be lacking.51,52 The prognosis is good.

Figure 144-4 Body louse, Pediculus humanus var. corporis, as it was obtaining a blood-meal from a human host.
(Courtesy Public Health Image Library, Centers for Disease Control and Prevention).
Orientia tsutsugamushi causes scrub typhus, or tsutsugamushi disease; it is transmitted by the bite of mite larvae.40 The disease occurs in Japan, eastern Australia, eastern Russia, China, and the Indian subcontinent, mainly in autumn and spring.53 Approximately 1 week after the bite, patients present with fever, headaches, and myalgias.54 An eschar may be observed in 50% of patients and is often associated with adjacent lymphadenopathy.54 The rash is macular, faint, and transient and can be missed.4 Neurologic symptoms are relatively common and vary from confusion to delirium and coma.54 Severe forms can progress to septic shock.55 Relapses can occur and are less severe than the first episode.55
Diagnosis
The leukocyte count can be within normal limits, but a leukopenia can be observed.56 Thrombocytopenia can occur and may be marked in severe cases. Anemia can also be present, especially when hemolysis is observed (frequently in patients with G6PD deficiency).56 Coagulopathy, with decrease in clotting factors (including fibrinogen) and prolonged coagulation times, may contribute to bleeding. C-reactive protein and hepatic enzyme levels can be increased.56 Hyponatremia and hypocalcemia, as well as increased lactate dehydrogenase and creatine phosphokinase levels, usually reflect the severity of the disease and organ involvement.56
The diagnosis of rickettsioses is based on serology.57 Rickettsial antibodies can be detected by several serologic tests which have different sensitivities and specificities: complement fixation,58 indirect hemagglutination,59 latex agglutination,60 enzyme-linked immunosorbent assay (ELISA),61 immunoperoxidase assay,62 and immunofluorescence assay (IFA).63 Not all serologic tests differentiate between immunoglobulins (ig)IgG and IgM or are specific enough for the diagnosis of different spotted fever–group Rickettsiae.64 IFA is regarded as the gold standard for serologic diagnosis of rickettsial infections; its sensitivity and specificity are highest among the diagnostic methodologies, and it is able to differentiate between IgG and IgM.60,64–66 Two sera samples should be tested because the early serum is often negative. A cutoff value of 1/64 for total immunoglobulins and 1/32 for specific IgM is usually required for the diagnosis.64 Cross-reactive antibodies have been observed with infections caused by Ehrlichia, Bartonella, Legionella, and Proteus. A cross-adsorption test is used to discriminate cross-reacting antibodies between two or more antigens, but the technique is limited by the large amount of antigen needed.64 Western immunoblot assay is the most specific and sensitive serologic assay and is used for epidemiologic purposes and confirmation of serologic diagnoses obtained by conventional tests.64
In skin biopsies, preferably from petechial lesions and eschar, the bacteria can be detected before seroconversion occurs. Skin biopsies can also be used for retrospective diagnosis.2,64 Immunofluorescence and immunoperoxidase techniques can be performed on frozen or fixed samples as well as on paraffin-embedded material.64
Skin biopsy specimens, peripheral white blood cells, or suspected arthropods may be used for polymerase chain reaction (PCR) diagnosis. PCR is a highly useful tool for the diagnosis of rickettsioses, but the sensitivity of the usual PCR amplification with clinical specimens seems to be variable.67 In an effort to improve rickettsial DNA detection and to avoid false-positive results, a new technique called suicide PCR has been introduced that is more sensitive and specific than traditional methods.67
The isolation of rickettsiae can be performed from human samples (decanted plasma or skin biopsies, ideally from the eschar) and from arthropods.64 Culture is restricted to specialized laboratories with biohazard and cell culture facilities. Usually, culture of rickettsiae takes 3 to 7 days.64 This technique is fundamental for the identification of new rickettsial pathogens.
Treatment
Doxycycline is the treatment of choice for rickettsioses.68 It can be prescribed in adults and children,69 but not in pregnant women and patients with allergy to tetracycline or related antibiotics. The fever typically subsides within 1 or 2 days after treatment is started; clinical improvement might be slower in complicated cases or critically ill patients, especially if they have multiple organ dysfunction.70 The treatment should be given orally, except in patients with gastric intolerance or coma, for whom it should be administered intravenously (IV).4 A single treatment of 200 mg of doxycycline in one day is sufficient for most of the rickettsioses (but not RMSF).4 For RMSF, scrub typhus, or the severe form of spotted fever, treatment duration is usually longer, and the recommendation is to continue doxycycline, 100 mg twice daily for 2 to 3 days after the patient becomes afebrile and until evidence of clinical improvement is noted, usually at least 7 days.4,70 The pediatric dose of doxycycline is 2.2 mg/kg body weight per dose administered twice daily (orally or IV) for children weighing less than 100 lbs (45.4 kg).70 R. akari and R. prowazekii infections are treated with doxycycline, 200 mg daily for 7 days, or with chloramphenicol as alternative treatment.4 Chloramphenicol, 50-75 mg/kg/d for 10 days, is the only available alternative to doxycycline in pregnant women and allergic patients.68 Chloramphenicol is available only in an IV form in the United States. Erythromycin has been used with success for murine typhus in several cases.44,71 Rifampin (600-900 mg daily) and azithromycin (500 mg daily) are alternative treatment for scrub typhus and can also be prescribed during pregnancy.72
Severely ill patients must be treated in intensive care units (ICUs). Fluid administration should be carefully monitored. Anemia and coagulation abnormalities should be corrected. Mechanical ventilation can be required in cases of respiratory distress. Hemodialysis may be required in patients with renal insufficiency. Antiepileptic drugs should be given to treat seizures. In cases of gangrene, amputation is sometimes necessary. Glucocorticoids have not proven beneficial.73
There is no current vaccination for rickettsial diseases, and prevention is based on the avoidance of tick, flea, and body lice bites.4 Lice are fragile, so changing and boiling clothes is effective. Repellents and/or protective garments can also be used. After possible exposure, ticks can be removed by forceps followed by skin disinfection.4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




