CHAPTER 12 Reperfusion Therapies for Acute ST Segment Elevation Myocardial Infarction
Historical Perspective
Thrombosis was implicated as the cause of acute myocardial infarction (MI) almost a century ago.1 The pathophysiology remained obscure, however, and as recently as 35 years ago most investigators believed that thrombosis was a secondary event.2 Clarity followed demonstrations by Chazov and colleagues3 and later Rentrop and coworkers,4 who showed angiographically that recanalization was achievable pharmacologically with favorable electrocardiographic (ECG) and clinical consequences. It then became progressively clear that ischemic injury could be attenuated by restoration of myocardial perfusion.5
Underlying this rapid paradigm shift was a hypothesis formulated by Braunwald that MI evolves dynamically, that the magnitude of irreversible injury sustained is related to the duration of ischemia, and that the clinical consequences of infarction are largely a reflection of the extent of irreversible injury sustained.6 It was postulated that reduction of myocardial oxygen requirements, enhancement of myocardial perfusion, or both when implemented within the first few hours after the onset of myocardial ischemia would mitigate the magnitude of irreversible injury sustained by the myocardium and would improve prognosis. Against this backdrop, the value of induction of reperfusion with pharmacologic agents, percutaneous coronary intervention (PCI), or both ultimately became established and resulted in marked improvements in prognosis. Before this paradigm shift had occurred, early (30-day) mortality from acute ST segment elevation myocardial infarction (STEMI) was greater than 30%. Presently, 30-day mortality is 7%, largely as a result of reliance on early reperfusion as the linchpin of therapy. This chapter addresses the developments responsible for this profound improvement in survival.
Coronary Occlusion and Reperfusion of Myocardium: Fibrinolysis and the Development of Fundamental Concepts Underlying Treatment
MI remains the leading cause of death in much of the Western world.7 Benefit attributable to reduction in myocardial oxygen requirements is modest. Early administration of intravenous β blockers elicited variable and limited reduction in mortality,8–11 perhaps, although inconsistently, attributable to reduction in infarct size.12–14 These observations are consistent with subsequent observations made in the COMMIT Trial,15 which showed reductions in the incidences of reinfarction and ventricular arrhythmia, but an increased incidence of cardiogenic shock. Beneficial effects of intravenous nitrates were seen with meta-analyses,16 but often not in individual trials. Before reperfusion became a mainstay of treatment, hospital mortality after acute MI was almost fourfold greater than it is today.17,18
The duration of coronary occlusion was shown to be a determinant of the extent of myocardial damage in laboratory animals in 1941.19 In the 1970s, it became clear that infarct size was a major determinant of prognosis.20,21 This discovery and the subsequent proof that coronary artery thrombosis was often the precipitating cause of infarction led to a focus on restoration of blood flow through the infarct-related artery with plasminogen activators.
Coronary Thrombosis and the Pathogenesis of Acute Myocardial Infarction
Although plaque rupture followed by coronary thrombosis is known to precipitate acute MI,22,23 its role has been debated extensively. Early autopsy studies of patients who died suddenly failed to show a high incidence of coronary thrombotic occlusion, perhaps because of antemortem or postmortem fibrinolysis. Although Herrick1 attributed fatal acute MI to a thrombotically occluded coronary artery in 1912, and plaque fissuring was implicated as causal in 1966,24 autopsy studies in the late 1970s did not show preponderant coronary thrombosis in patients who had died of acute MI.2,25 These studies led to the speculation that coronary thrombosis was a consequence, rather than the underlying cause, of acute MI.25
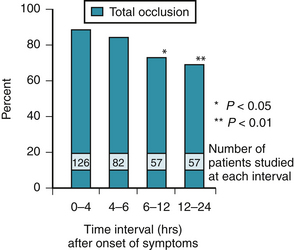
In 1980, DeWood and colleagues26 reported the results of coronary angiography performed early after the onset of acute transmural MI. This pivotal study showed a high prevalence of total and subtotal coronary occlusion, particularly within the first 4 hours after the onset of symptoms. The study also showed a high incidence of spontaneous recanalization over time. Within 4 hours of symptom onset, 87% of infarct-obstructed arteries were completely occluded. The prevalence of coronary occlusion was only 65%, however, 12 to 24 hours after onset (Fig. 12-1). When patients with subtotal occlusion of the obstructed artery were included, the prevalence of angiographically demonstrable coronary thrombosis in the first 4 hours was 98%. Angioscopic data from a smaller number of patients confirmed that thrombi are almost universally prevalent at the time of occurrence of acute STEMI.27
Efforts to reduce mortality soon focused on rapid restoration of blood flow in thrombotically occluded coronary arteries. It became clear that dissolution of clots postmortem28 explained the failure of earlier autopsy studies to detect the high prevalence of thrombi in victims of sudden cardiac death after acute MI. It is now known that the use of plasminogen activators can reduce early hospital mortality of patients with acute MI to 2% to 6% when early administration and optimal dosing of clot-selective agents are employed.29–31
Coronary Thrombolytic Agents: Proving the Value of Reperfusion
The maintenance of fluidity of blood depends on a complex balance between thrombosis, thrombolysis, and counter-regulation by inhibition of both processes. In vessels supplying regions of the heart undergoing acute MI, the rupture or fissuring of an underlying atherosclerotic plaque leads to thrombosis with exposure of the blood to the procoagulant effects of exposed type I collagen, von Willebrand factor, and tissue factor in the vessel wall. Activation of platelets accompanying the vascular injury accelerates ongoing thrombosis.32,33 Thrombin and fibrin generated by the coagulation cascade may undergo concomitant or subsequent lysis resulting from activation of the fibrinolytic system and conversion of the zymogen plasminogen to the active serine protease, plasmin, by the circulating plasminogen activators, tissue plasminogen activator (t-PA) or urokinase plasminogen activator (UK). These key components of the fibrinolytic system had been identified by 1950,28,34 well before acceptance of coronary thrombosis as the crucial step leading to transmural MI.
Circulating plasminogen is activated endogenously by t-PA and UK, resulting in the generation of plasmin that leads to degradation of fibrin to form soluble fibrin degradation products. Such products, activation peptides, and enzyme inhibitor complexes can be measured quantitatively as markers of fibrinolysis. Examples include fibrinopeptide A, prothrombin 1.2 and other prothrombin fragments, a fragment of the fibrinogen β chain (β 1-42), and complexes of thrombin-antithrombin.35–38 A specific degradation product of cross-linked fibrin, a fragment known as D-D dimer, reflects degradation of fibrin associated with fibrinogenolysis accompanying a systemic lytic state seen whenever plasminemia is present.39,40 Fibrinolysis is inhibited by circulating α2-antiplasmin, an inhibitor of plasmin, and by inhibitors of plasminogen activators in blood, primarily plasminogen activator inhibitor 1 (PAI-1).41 The fibrinolytic system is shown schematically in Figure 12-2.
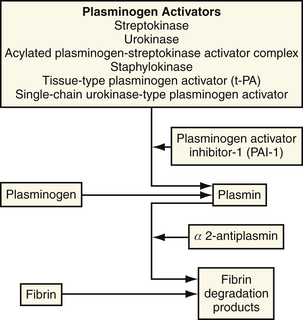
Figure 12-2 Regulation of the plasma fibrinolytic system.
(From Collen D: Towards improved thrombolytic therapy. Lancet 1993;342:34.)
Plasminogen activators can paradoxically promote thrombosis. First-generation plasminogen activators, agents that are not fibrin-selective or clot-selective, such as streptokinase (SK), UK, and anisoylated plasminogen activator complex (APSAC), convert circulating and clot-bound plasminogen indiscriminantly to plasmin. Rapid depletion of plasma α2-antiplasmin occurs with plasminemia, which may generate thrombin from precursors and activation of the coagulation cascade.42,43 Procoagulant effects of plasminemia reflect activation of the so-called extrinsic and intrinsic coagulation pathways.44–46 The thrombin activity induced may activate platelets and lead to reocclusion after initially successful clot lysis.47 Plasminemia also can lead to a phenomenon we have called plasminogen steal, in which conversion of circulating plasminogen to plasmin induces a leaching of fibrin-associated plasminogen into blood through mass action.48 The consequent reduction in clot-associated plasminogen diminishes the intensity of fibrinolysis and reduces the efficacy of plasminogen activators.
Thrombolytic Agents
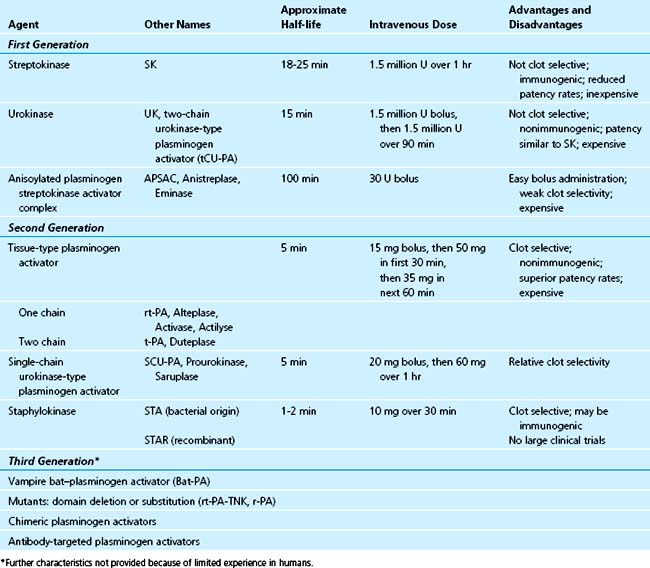
The available thrombolytic agents are plasminogen activators. These agents function as proteases that directly or indirectly hydrolyze a single peptide bond (Arg561Val562) on the inactive substrate molecule, plasminogen, to form the active serine protease enzyme, plasmin. Plasmin is responsible for the degradation of fibrin and diverse other proteins, with consequent dissolution of intravascular thrombi. So-called first-generation agents (non–fibrin-selective) include SK, UK, and APSAC. Second-generation and later generation (fibrin-selective) agents include t-PA, single-chain urokinase-type plasminogen activator (scu-PA), staphylokinase, and others, including molecular variants of t-PA such as TNK t-PA (tenecteplase). Agents that are relatively fibrin-specific, such as t-PA, produce less depletion of fibrinogen, less plasminemia, and less depletion of α2-antiplasmin than that seen with non–fibrin-specific agents such as SK. Fibrin-bound plasmin generated by clot-selective agents is not susceptible to rapid inhibition by α2-antiplasmin in the blood, in contrast to the circulating free plasmin that is neutralized promptly until α2-antiplasmin is depleted.28 Table 12-1 summarizes the nomenclature, classification, and mechanism of action of several agents.
Non–Fibrin-Selective Agents
Streptokinase
SK is a protein present in numerous strains of hemolytic streptococci. It is a single-chain polypeptide that lacks the serine residue required for enzymatic activity, but it can activate plasminogen indirectly through an intricate, three-step process.49,50 Initially, SK forms an equimolar complex with plasminogen, resulting in exposure of the active site on the plasminogen molecule, which leads to the enzymatic conversion of plasminogen to plasmin by the exposed active site. The plasminogen-SK complex is converted to various, differentially cleaved plasmin-SK complexes,51 some of which are less active or more rapidly cleared than the SK-plasminogen complex, but can still convert plasminogen to plasmin.52
Because SK is not fibrin-selective, extensive conversion of circulating plasminogen to plasmin occurs with subsequent depletion of fibrinogen, plasminogen, and factors V and VIII from the bloodstream. The accumulation of by-products of fibrinogen breakdown products, depletion of circulating α2-antiplasmin, and hyperplasminemia that occur constitute a systemic lytic state. A systemic lytic state occurs with all therapeutically effective doses of non–fibrin-selective plasminogen activators given intravenously. It is less intense with low-dose intracoronary administration.53
The circulating half-life of SK is approximately 18 to 25 minutes. Depletion of fibrinogen to less than 50% of baseline values persists for approximately 24 hours, however. Because of the foreign nature of the protein and the near-universal human exposure to the bacterial sources of the agent (beta-hemolytic streptococci), administration of SK is complicated by inhibition of the administered drug by circulating IgG antibodies and problems of immunogenicity and attendant allergic reactions. In most humans, approximately 350,000 U of SK is necessary to neutralize circulating antibodies, but the range varies widely.50–54 With the conventional clinical dose of 1.5 million U, pretreatment circulating antibody levels do not correlate with subsequent patency rates or clinical outcome.55
After administration of SK, anti-SK titers rise quickly and are virtually universally elevated within 5 days, remaining above baseline for 30 months.56,57 Consequently, repeated administration of SK is impractical and is not recommended.
The unfavorable profile of adverse reactions associated with SK (presumably attributable to plasmin-mediated activation of kininogen) limits clinical use of this agent to some extent. The overall incidence of hypotension ranges from 10% to 40%.50,58 It is highest with rapid infusion.59 Severe hypotension requiring pressor agents or fluids occurs in 5% to 10% of patients. Other allergic reactions reported include fever, chills, urticaria, rash, flushing, and muscle pain. In the large-scale ISIS-2 and GUSTO-I trials, the incidence of minor allergic reactions was 4% to 6%.30,60 The incidence of anaphylactic shock is low, occurring in 0.7% of patients in GUSTO-I.30
The conventional dose of SK is 1.5 million U administered over 1 hour by intravenous infusion. This regimen was described in 1983,61 and was used successfully in the GISSI-1 trial in 1986.62 More rapid administration can lead to a high incidence of hypotension and should be avoided.
Anisoylated Plasminogen Streptokinase Activator Complex
APSAC is a first-generation plasminogen activator that is a complex of human Lys-plasminogen and SK, with acylation of the plasminogen designed to block the active site until deacylation occurs slowly in vivo.50 It is administered by intravenous bolus injection.63 Deacylation occurs in the circulation, but the complex manifests no fibrin specificity.64 Its half-life in the circulation is approximately 100 minutes. Because SK is the major component of APSAC, the drug is immunogenic and has a side-effect profile similar to that for SK. Similar induction of anti-SK antibodies56 occurs, precluding repeated administration. Its fibrinolytic properties are virtually identical to the fibrinolytic properties of SK. The recommended dose in patients with acute MI is 30 U, given as an intravenous bolus. The nadir in plasma fibrinogen and α2-antiplasmin is comparable to that seen with 1.5 million U of SK.50
Urokinase
UK, an endogenous trypsin-like enzyme, is a direct plasminogen activator. It is present in urine and occurs in two forms in blood and tissue: a high-molecular-weight form and a low-molecular-weight form.65 Its precursor is scu-PA, which is enzymatically inactive. Cleavage of scu-PA by plasmin yields high-molecular-weight UK, a two-chain, disulfide-linked molecule that lacks fibrin specificity and indiscriminantly activates circulating and fibrin-bound plasminogen, with associated depletion of α2-antiplasmin. It degrades fibrinogen and other plasma proteins and induces a systemic lytic state comparable to that seen with SK.
UK has a plasma half-life of approximately 15 minutes. Primary clearance is in the liver, with a small fraction (3% to 5%) cleared by the kidney.54 UK has been used commonly in patients undergoing interventional procedures for coronary or peripheral vascular disease and in patients with pulmonary embolism. It is nonimmunogenic and can be administered as an intravenous bolus66 or by infusion. The recommended dose for acute MI is a bolus of 1.5 million U followed by 1.5 million U given over 90 minutes.
Relatively Fibrin-Selective Agents
Tissue Plasminogen Activator
t-PA is an endogenous serine protease synthesized and secreted by human vascular endothelium and numerous other types of cells. When t-PA was isolated from a human (Bowes) melanoma cell line,67 definitive evaluation of its biochemical and pharmaceutical features became possible. It was first given to patients with acute MI in 1984.68 The cloning and expression of the human t-PA gene in Escherichia coli in 1983 by Pennica and colleagues69 and the development of recombinant t-PA led to administration of t-PA to patients.70
The plasma half-life of t-PA is only 5 minutes. Fibrinolytic activity persists on and within clots for 7 hours, however.71 t-PA is metabolized by the liver. It is inhibited in plasma by PAI-1 and other inhibitors. PAI-1, the “fast-acting inhibitor,” was characterized in the early 1980s.72–74 Infused t-PA rapidly saturates circulating PAI-1, and subsequently, circulating free t-PA complexes more slowly with inhibitors such as C-1 esterase inhibitor and α2-antiplasmin.74,75
An important advantage of t-PA compared with SK is its affinity for fibrin-bound plasminogen through sites in the NH2-terminal (heavy) chain.50 In the absence of fibrin, t-PA is a weak activator of plasminogen. When fibrin is present, however, activation of plasminogen associated with it is rapid and intense. Clinically, conventional doses of t-PA induce some degradation of circulating fibrinogen (to approximately 50% of baseline) and some elevation of concentrations of fibrinogen degradation products. The relative fibrin specificity of t-PA accounts for the more rapid clot lysis seen with t-PA compared with SK.76 Because the specificity for fibrin is not absolute, however, doses used clinically elicit degradation of circulating fibrinogen, albeit less than that seen with SK.77
t-PA is available commercially as alteplase, which is primarily single-chain t-PA. Duteplase, a primarily double-chain t-PA with a different primary structure and different properties, was used in the ISIS-3 study, but is not commercially available. The two agents differ considerably with respect to risk of toxicity and probably differ in therapeutic efficacy.50,78–80
In early clinical trials, the intravenous dose of t-PA was 60 mg in the first hour, with an initial 6-mg bolus, followed by 20 mg/hr for the next 2 hours. The total dose, 100 mg, was selected in part because higher doses had been associated with intracerebral hemorrhage (1.9% incidence with 150 mg administered over 3 hours).81 Neuhaus and coworkers82 introduced “front-loaded” dosing (i.e., 15-mg bolus with 50 mg given by infusion over the first 30 minutes, followed by 35 mg over the next 60 minutes). This regimen was associated with a 91% patency rate at 90 minutes, and it has now been approved by the U.S. Food and Drug Administration.
Third-Generation Fibrinolytic Agents
Staphylokinase
The profibrinolytic properties of staphylokinase, a protein elaborated by strains of Staphylococcus aureus, have been recognized for more than 40 years.83 Results in early studies in animals were not promising,84,85 and enthusiasm for this agent soon waned. A recombinant DNA-synthesized variety (STAR) has been developed. Compared with SK, it is more powerful and fibrin selective.86 STAR, similar to SK, is not an enzyme. It forms an active proteolytic complex in 1:1 stoichiometry with plasminogen. Although immunogenic, it is a remarkably fibrin-selective fibrinolytic agent.87 Its thrombolytic potency with platelet-rich arterial thrombi is impressive.88 Compared with SK, STAR induces more frequent and more persistent arterial recanalization. In a pilot study, Collen and Van de Werf89 showed successful coronary recanalization in four of five patients with evolving acute MI with 10 mg of intravenous recombinant staphylokinase. Plasma fibrinogen and α2-antiplasmin were not significantly decreased, and allergic reactions did not occur. Neutralizing antibodies to STAR were detected in plasma consistently within 14 to 35 days, however. Variants with less immunogenicity are being pursued (2004 D Collen, personal communication).
Tissue Plasminogen Activator Mutants
Hundreds of deletion, insertion, substitution, and combination mutants of wild-type t-PA have been synthesized. One, reteplase, initially called r-PA (also known as BM 06.022), has been studied in clinical trials and marketed as Retavase. Reteplase lacks the kringle 1 domain, resulting in a prolonged half-life and facilitating bolus administration.90 It induces coronary recanalization rapidly in dogs,91 and has elicited early vessel patency in initial clinical studies. Early reocclusion has been encountered, however, implying the potential need for a double-bolus dosing regimen.92,93 It is not as fibrin-selective as wild-type t-PA.
Mutants of t-PA with prolonged half-lives have often exhibited reduced thrombolytic efficacy.94 Generally, they have not seemed to be superior to wild-type t-PA.95 One exception is a so-called triple mutant of t-PA, referred to as TNK t-PA (tenecteplase).95 The acronym refers to the three amino acid substitutions that differentiate TNK t-PA from wild-type t-PA. They result in reduced inhibition of the plasminogen activator by PAI-1, prolongation of half life as a result of decreased uptake by the reticuloendothelial system mediated by mannose receptors, and consequent efficacy after bolus injection. TNK t-PA seems to induce reperfusion more rapidly than t-PA in patients treated within 3 hours after onset of symptoms.96
Mortality Benefit of Pharmacologic Reperfusion: Clinical Trials of Coronary Thrombolysis
Early Observations
Recanalization trials performed in the late 1970s and early 1980s provided vital information by angiographically documenting relief of thrombotic coronary occlusion induced by plasminogen activators. Initially, coronary thrombolysis was performed with intracoronary administration of plasminogen activators. This approach showed the feasibility of clot lysis and induction of recanalization.3,4 Rentrop and colleagues,97,98 using intracoronary SK, showed improved cardiac function and alleviation of chest pain accompanying recanalization compared with intracoronary nitroglycerin alone or conventional therapy. The Western Washington randomized trial99 substantiated the efficacy of intracoronary SK in lysing coronary thrombi, with favorable effects on mortality. High rates of recanalization with intravenously administered t-PA were observed soon thereafter.68,100,101
Intracoronary administration of SK seemed to be more capable of inducing prompt recanalization than SK administered intravenously.102 Logistic constraints on the availability of immediate cardiac catheterization, time delays, increased costs, and increased risk limited enthusiasm, however, for intracoronary administration of plasminogen activators as primary therapy for patients with acute MI. The appeal of the intravenous route was considerable. Nevertheless, observations in a study in which intracoronary SK was administered after PCI and stenting with apparently increased myocardial perfusion compared with the perfusion seen in the absence of SK may rekindle interest in the intracoronary administration of fibrinolytic drugs.103
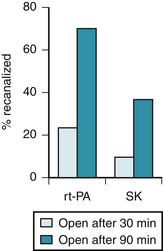
The TIMI phase I trial compared intravenous t-PA (80 mg over 3 hours) with intravenous SK (1.5 million U over 1 hour).104 After 90 minutes, twice as many occluded arteries had been opened by t-PA (62%) as by SK (31%) (Fig. 12-3). The superiority of t-PA was evident, regardless of the interval between symptom onset and treatment. t-PA induced more rapid and more frequent clot lysis in the infarct-affected artery.
Patency Trials
Patency trials delineate angiographically defined patency at specified intervals after treatment. Prompt treatment seemed to maximize benefit in the multicenter GISSI-1 mortality trial in 1986.62 Patency trials are characterized by an unavoidable lack of certainty, however, regarding the actual incidence of thrombotic occlusion before therapy, and the inclusion of patients with spontaneous thrombolysis. Nevertheless, such trials were helpful in comparing diverse agents with respect to overall patency. Angiography was required because noninvasive criteria of reperfusion, including relief of chest pain, ECG changes, early washout of enzymes, and arrhythmias, did not reflect actual incidences of recanalization.105
It soon became clear that the extent and persistence of restoration of flow required to salvage ischemic myocardium were pivotal. Angiographic classifications based on the transit of contrast media through an infarct-related occluded vessel after treatment with plasminogen activators provided useful indices. One set of criteria employed frequently was established in the TIMI phase I trial in 1985 (Table 12-2).106 It classified coronary flow from TIMI grade 0 (no flow) to TIMI grade 3 (brisk flow of contrast material). TIMI grade 1 (minimal flow of contrast material) and TIMI grade 2 (delayed flow of contrast material) were seen in patients with residual stenosis, coronary vasospasm, ongoing thrombosis, and the no-reflow phenomenon, in which forward flow is restricted, despite a patent vessel, by microvascular stasis downstream as a result of leukocyte and platelet plugging, vasoconstriction, or tissue and cellular edema.
Table 12–2 Angiographic Definitions of Perfusion from the TIMI Phase I Trial
| Grade 0 (no perfusion) | There is no antegrade flow beyond the point of occlusion |
| Grade 1 (penetration without perfusion) | Contrast material passes beyond area of obstruction, but “hangs up” and fails to opacify the entire coronary bed distal to the obstruction for the duration of the cineangiographic filming sequence |
| Grade 2 (partial perfusion) | Contrast material passes across the obstruction and opacifies the coronary bed distal to the obstruction. Rate of entry of contrast material into the vessel distal to the obstruction, its rate of clearance from the distal bed, or both are perceptibly slower than entry into or clearance from comparable areas not perfused by the previously occluded vessel, such as the opposite coronary artery or the coronary bed proximal to the obstruction |
| Grade 3 (complete perfusion) | Antegrade flow into the bed distal to obstruction occurs as promptly as antegrade flow into the bed proximal to the obstruction, and clearance of contrast material from the involved bed occurs as rapidly as clearance from an uninvolved bed in the same vessel or the opposite artery |
From Chesebro JH, Knatterud G, Roberts R, et al: Thrombolysis and Myocardial Infarction (TIMI) trial, phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Circulation 1987; 76:143.
Myocardial contrast echocardiography has been used also to assess the adequacy of restored perfusion. In 39 patients with acute anterior MI in whom recanalization was induced by percutaneous transluminal coronary angioplasty (PTCA) or thrombolysis, subsequent delivery of microbubbles into the coronary circulation followed by two-dimensional surface echocardiography identified nine patients in whom microcirculatory reflow was absent despite coronary patency.107 Compared with the remainder of the study group, these patients exhibited significantly reduced segmental and global left ventricular function indicative of suboptimal myocardial salvage.
Most early patency trials employed angiographic end points to delineate patency 90 minutes after the administration of a thrombolytic agent. Patients with TIMI grade 2 or TIMI grade 3 were considered together in delineating overall patency incidence. Even when no thrombolytic agent is given, patency rates range from 9% to 29% in the 0- to 90-minute interval.100,104,108–112 Considerable “catch up” occurs (i.e., patency attributable to endogenous fibrinolysis), as judged from results of arteriography performed later. Patency rates range from 36%113 to 78%114 3 to 21 days after MI in patients not treated with plasminogen activators.115,116
Despite the higher patency rates seen with t-PA compared with SK, results of early megatrials (GISSI-1, ISIS-2)60,62 comparing the two agents did not show differences in mortality. The apparent lack of coupling between patency and mortality in these early trials fueled speculation that benefits did not depend on early opening of an infarct-occluded artery, regardless of how quickly coronary recanalization was achieved. Although an infarct-related occluded artery rendered patent late may confer some benefits unrelated to salvage of jeopardized myocardium, such as altered ventricular remodeling and improved electrical stability,117,118 the reduction of mortality of patients treated with thrombolytic agents depends primarily on the rapidity and persistence of recanalization. A striking 50% reduction in mortality rates occurred for patients treated within 1 hour of symptom onset in the GISSI-160 and ISIS-262 trials, with the benefit less striking but still evident in patients treated within 3 to 4 hours. A 1% mortality rate was seen in the MITI project31 for patients with documented MI treated with t-PA within 70 minutes of the onset of symptoms. In many patients, no late scintigraphic evidence of irreversible injury was observed, consistent with extensive and perhaps complete myocardial salvage. The failure to recognize the dependence of mortality reduction on early patency in the megatrials previously mentioned probably reflects the late time to treatment (and obviation of benefit) for many patients, and the failure to employ the adequate conjunctive anticoagulation needed to sustain initially induced patency.
The magnitude of restoration of flow seems to be a major determinant of benefit. Patency may be an inadequate term to describe the full impact of any given reperfusion therapy: TIMI grade 2 and TIMI grade 3 flow have different implications. Patients with delayed transit of contrast material in the infarct-affected artery (TIMI grade 2 flow) may not be exhibiting optimal or adequate recanalization. The TEAM-2 study analyzed data with respect to flow in patients treated with intravenous APSAC or SK.119 When TIMI flow grades were considered with respect to enzymatic and ECG markers of infarct size, no statistically significant difference was seen for TIMI flow grades 0, 1, or 2. Better outcomes were seen, however, with TIMI grade 3 flow.
In a retrospective analysis of four multicenter German studies (907 patients), TIMI grade 2 flow was associated with a mortality similar to that of patients with persistently infarct-occluded vessels.120 The in-hospital mortality rate of patients with TIMI grades 0 and 1 was 7.1%. With TIMI grade 2, it was similar (6.6%). With TIMI grade 3, the mortality rate was significantly lower (2.7%). The GUSTO-I angiographic study provided another comparison. Lack of patency (TIMI grade 0 or 1) was associated with the highest mortality rate (8.9%). Traditionally defined patency (TIMI grades 2 and 3) was associated with a lower mortality rate (5.7%; P = .004).121 The mortality for patients with TIMI grade 2 flow was 7.4%, and even lower (4.4%) for patients with TIMI grade 3 flow (P = .08).
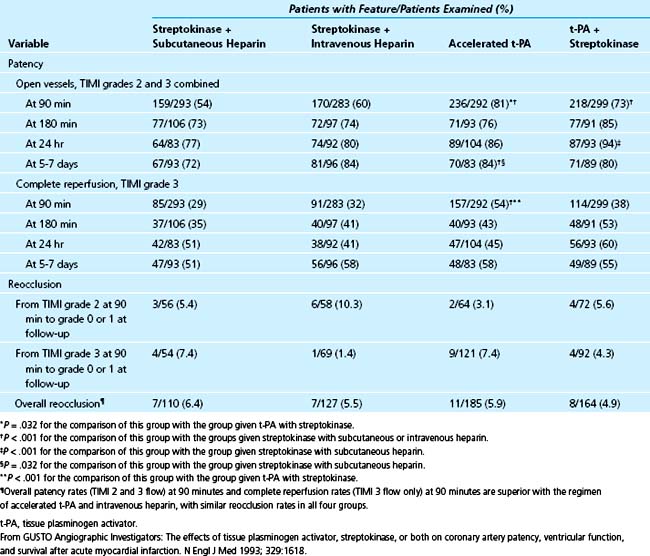
Front-loaded regimens of t-PA seem to be superior in terms of induction of TIMI grade 3 flow compared with other agents. The GUSTO-I angiographic trial directly compared SK, t-PA, and the combination of t-PA and SK (Table 12-3).121 Front-loaded t-PA was associated with complete reperfusion at 90 minutes (TIMI grade 3) in 54% of patients. With SK alone, complete reperfusion occurred in less than 32% of patients (29% with subcutaneous heparin and 32% with intravenous heparin). In patients given t-PA and SK, reperfusion occurred in 38%. Similar rates of complete reperfusion at 60 minutes had been seen in earlier trials, with patency and TIMI grade 3 flow ranging from 54% to 62%29,82,122 with front-loaded t-PA compared with 40% with intravenous APSAC29 and 40% with the standard dose of t-PA.122
Table 12–3 Results of the GUSTO-1 Angiographic Study: Patency and Reocclusion of the Infarct-Occluded Artery According to Treatment Group
One straightforward intervention would undoubtedly decrease mortality and increase the efficacy of coronary thrombolysis markedly. Fresh clots lyse much more rapidly than older ones in which fibrin cross-linking has proceeded.123 Intervention within 30 to 60 minutes is likely to be particularly beneficial because more myocardium would remain viable and amenable to salvage, and because clot lysis would be much more rapid and complete. The rapidity with which patients are treated should be maximized. Current American College of Cardiology (ACC)/American Heart Association (AHA) guidelines recommend the “earliest possible application of therapy,” and refer to therapy with fibrinolytic agents in the setting of STEMI with symptoms within 12 hours and ECG changes of 0.1 mV in two contiguous leads or new left bundle branch block as a class 1a recommendation.
Left Ventricular Function and Pharmacologic Induction of Reperfusion
Left ventricular contractile function as an end point in trials of coronary thrombolysis requires exceptionally careful analysis. Early placebo-controlled trials of coronary thrombolysis in which left ventricular ejection fraction was a primary end point showed variable but generally consistent group improvement in left ventricular function and indices of infarct size (e.g., enzymatic, scintigraphic) in patients treated with thrombolytic agents.99,114,124–130 Similar results were seen with global and regional measures of ventricular function.124,129 Improvement in ejection fraction has generally been greatest in groups of patients with anterior infarction, consistent with the large amount of left ventricular muscle supplied by the left anterior descending coronary artery. Patients with inferior infarction have shown improved regional and global left ventricular function as well.127,131 Analysis of results in the ISAM study indicated that the patency of an infarct-occluded artery at 1 month was associated with good left ventricular function regardless of treatment (active or placebo) and the vessel involved.132
A key observation was made by Van de Werf,133 who recognized that effective thrombolysis enhances survival of patients with severely reduced left ventricular function who would have otherwise died. Lower ejection fractions are observed in the entire group of treated patients. In essence, the low ejection fractions in survivors with severe insults account for the apparent paradox that becomes prominent when large reductions in early mortality are achieved. This paradox has been called the ventricular function–mortality paradox. When it is considered along with methodologic limitations, it becomes clear that, contrary to speculation by some authors,134 assessment of ventricular function in groups is an ambiguous end point for comparing different agents or delineating the efficacy of specific conjunctive and adjunctive regimens. Conversely, sequential measurement of regional ventricular function in individual patients provides a more valid measure of benefit conferred by early and sustained recanalization.
Despite such limitations, correlations between patency and improved function have been striking. In more than 1200 patients enrolled in the five phases of the TAMI trials, TIMI grade 2 flow was associated with a higher incidence of recurrent ischemia and congestive heart failure, and with reduced improvement in global and regional left ventricular function compared with TIMI grade 3 flow.135 When patients were evaluated according to TIMI flow grades regardless of the treatment used, patients with TIMI grade 3 had more preservation of regional wall motion, lower end-systolic volume indices, and higher left ventricular ejection fraction values than patients with TIMI flow grades 0, 1, and 2 (Table 12-4) as judged from 90-minute and 5- to 7-day angiography. The results are consistent with the fact that myocardial necrosis is virtually complete in 4 to 6 hours.117 The investigators underscore the critical importance of the rapidity and completeness of recanalization.
Table 12–4 Association between Patency Grade and Measures of Left Ventricular Function from the GUSTO Trial
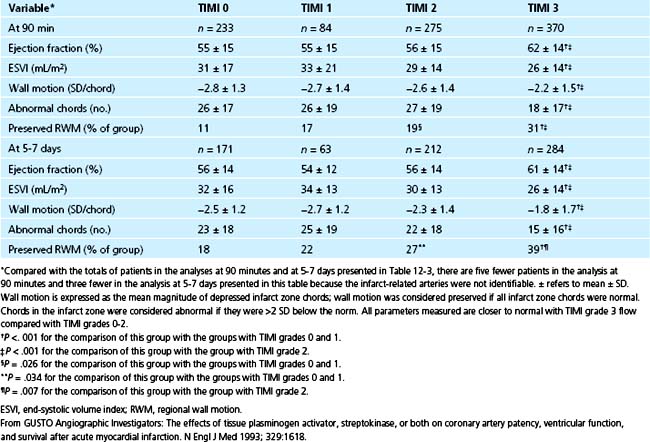
The TAMI-6 trial evaluated effects of late myocardial reperfusion (6 to 24 hours) induced by thrombolysis with intravenous t-PA (standard dose with a weight-adjusted regimen) or PTCA in patients with acute MI.136 Compared with placebo-treated patients, patients subjected to late intervention exhibited no difference in ejection fraction or infarct zone regional wall motion at 1 or 6 months. Compared with t-PA, placebo was associated with a significantly greater end-diastolic volume at 6-month follow-up (P = .006). Late thrombolysis or angioplasty exerted no benefit in terms of systolic function despite some prevention of cavity dilation in patients treated with thrombolytic agents. The observations are consistent with the view that early and complete recanalization salvages myocardium, improves systolic function, and reduces mortality.
Pivotal Placebo-Controlled Trials
Results of early, placebo-controlled trials showed consistent reduction of mortality despite differences among them with respect to entry criteria, thrombolytic agents, “conventional care,” and adjunctive therapy. In 1986, the landmark GISSI-162 trial showed a reduction in the overall 21-day mortality rate from 13% to 10.7% for 11,806 patients treated with intravenous SK rather than the usual treatment at that time. The trial documented a 47% reduction in mortality rates for patients treated with SK within 1 hour of symptom onset. Later studies of t-PA (ASSET, ECSG-5) showed analogous mortality reductions compared with placebo.114,137 Beneficial effects on survival with APSAC were seen in the AIMS trial,138 with a 47% reduction in the 30-day mortality rate for the treatment group leading to early termination of this placebo-controlled trial.
The largest of all the early placebo-controlled trials was ISIS-2.60 This trial randomly assigned 17,187 patients with acute MI to treatment with intravenous SK, oral aspirin, both, or neither. The 2 × 2 factorial design substantiated a reduction of mortality for patients treated with SK. The effects of aspirin alone were comparable. In retrospect, it seems clear that aspirin was reducing mortality in a subset of patients with unstable angina lumped with MI patients in this study, which failed to include a qualifying ECG as an entry criterion. The enrollment window from the onset of symptoms was 24 hours. As in GISSI-1, maximal benefit was seen in patients treated early (<4 hours from symptom onset). The smaller reduction in the 5-week mortality for patients treated in the 5- to 24-hour interval (P = .004) may have reflected prevention of MI in some patients with unstable angina or misidentification of the time of onset of MI in view of the omission of a qualifying ECG. Mortality reduction for patients treated in the interval 12 to 24 hours after symptom onset was not significant.
Time to treatment has been evaluated in the LATE study, in which 5711 patients presenting with acute MI that occurred 6 to 24 hours earlier were randomly assigned to intravenous t-PA or placebo.139 Treatment within 12 hours of symptom onset was associated with a 26% reduction of mortality for patients given t-PA. In patients treated from 12 to 24 hours, no benefit was evident. The EMERAS140 study compared intravenous SK with placebo in 4534 patients treated within 6 to 24 hours of symptom onset. In the 12- to 24-hour group, no benefit was shown, but some benefit (not significant) was implicated in the 6- to 12-hour group. This result may have reflected some misclassification of the actual time of onset of infarction or consequences of interruption of stuttering infarcts.
The apparently greater benefit seen with t-PA compared with SK in the LATE and EMERAS trials may reflect more effective lysis by t-PA of aged thrombi. Alternatively, more rapid lysis by t-PA than with SK may be responsible. A meta-analysis of more than 50,000 patients suggested that mortality can be reduced in patients treated within, but not beyond, 12 hours.141 The most compelling evidence indicates that the benefits of fibrinolytic induction of recanalization are minimal if it is not accomplished early, optimally within a few hours after onset of symptoms.
Mortality in Direct Comparison Trials
In 1992, the GISSI-2142 trial reported no difference in the mortality rates of 12,490 patients treated with intravenous SK compared with standard-dose t-PA (alteplase, single-chain t-PA). In contrast to most smaller trials in the United States, patients were not given protocol-mandated intravenous heparin; patients in the heparin arm were given 12,500 U subcutaneously beginning 12 hours after the onset of infusion of the fibrinolytic drug. The overall mortality of 8.8%, although lower than that seen earlier in GISSI-1, was considerably higher than the mortality rate in the pooled data (5.6%) from numerous trials with t-PA. Neither SK nor t-PA seemed to have been tested under conditions in which benefit could be optimal. The lack of intravenous heparin and the late time to treatment seem to contribute to this phenomenon. ISIS-3143 compared SK with t-PA (duteplase, double-chain t-PA) and APSAC in 41,299 patients. As in GISSI-2, ISIS-3 employed subcutaneous heparin (in 50% of the patients) at a dose of 12,500 U begun 4 hours after enrollment. No difference in mortality could be ascribed to any of the strategies. Combined results in the GISSI-2 trial for the overall 35-day mortality rate showed identical, high (10%) values for patients treated with SK and patients treated with t-PA.143
Viewed superficially, the results of these megatrials seem to conflict with the results of mechanistic trials that had clearly linked coronary recanalization with improved survival. The lack of adequate anticoagulation seems to be one major factor that contributed to high mortality rates and a lack of difference between t-PA and SK. The subcutaneous heparin regimen used does not lead to adequate anticoagulation in virtually any patient in the first 24 hours because of binding of heparin to endothelial cell binding sites and slow absorption.144
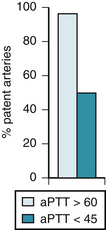
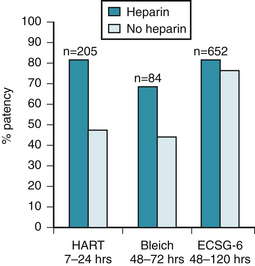
The need for administration of heparin with t-PA has been underscored by results in HART.145 The investigators randomly assigned 205 patients with acute MI to treatment with t-PA, aspirin, and intravenous heparin or t-PA and aspirin alone. Although 90-minute patency is the same (79%) with t-PA with and without heparin, as shown in the TAMI-3 trial,146 the patency rates between 7 hours and 24 hours in HART were 82% in the heparin group and only 52% in the aspirin group (P < .0001). A high incidence of reocclusion occurred when heparin was omitted. Analysis of the level of anticoagulation showed that patients with activated partial thromboplastin times (aPTTs) longer than 60 seconds had patency rates of 95%, in contrast to the dramatically lower late patency rate of 45% for patients with aPTTs less than 45 seconds (Fig. 12-4).147
Analogous results were obtained by the European Cooperative Study Group.148 The patency rate was higher for patients treated with t-PA and heparin and highest for patients with optimal anticoagulation. The same result was seen by Bleich and colleagues149 and in the ECSG-6 study.150 A consistent increase in persistence of coronary patency (from 7 to 120 hours after treatment) in patients undergoing coronary thrombolysis with t-PA accompanied coadministration of heparin, as shown in Figure 12-5. In view of the inadequate rapidity and magnitude of anticoagulation induced by the subcutaneous heparin dosage used in GISSI-2/International trial142,151 and ISIS-3,143 the high mortality rate and lack of fibrinolytic drug-dependent differences in ISIS-3143 and the GISSI-2/International trials142,151 are probably attributable to a design that led to substandard rates and rapidity of coronary recanalization and to a failure to maintain patency (particularly in the t-PA group), rather than to a lack of dependence of benefit and survival on early recanalization.
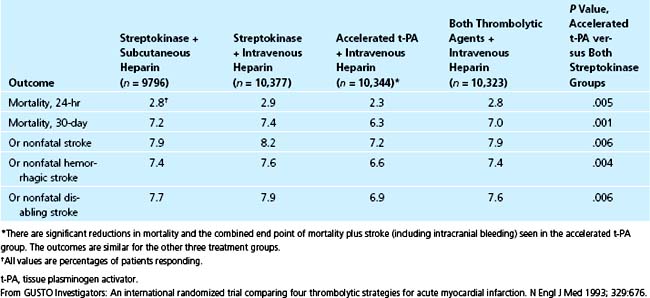
Because of allegations regarding the efficacy of combinations of fibrinolytic agents,152–154 and the need to delineate the relative merits of conjunctive anticoagulation, the GUSTO-I trial was implemented to compare four different regimens in 41,021 patients: SK with subcutaneous heparin, SK with intravenous heparin, front-loaded (also called accelerated) t-PA with intravenous heparin, and a combination of SK and t-PA with intravenous heparin.30 The 30-day mortality was lowest with front-loaded t-PA and intravenous heparin (6.3%) and significantly less than that with combination therapy (7%), SK and subcutaneous heparin (7.2%), and SK and intravenous heparin (7.4%) (Table 12-5). Reduction of mortality directly depended on the rapidity and adequacy of recanalization.121 Front-loaded t-PA was associated with fewer allergic reactions, less hypotension, less overall bleeding, and a lower incidence of recurrent ischemia, reinfarction, and diverse cardiac complications than the other regimens.30 The incidence of reocclusion was no higher than that with the other regimens.121 Overall, front-loaded t-PA led to more rapid and complete recanalization and an increase in the combined end point of survival without a stroke, equivalent to 10 lives saved per 1000 patients treated compared with either SK regimen.
Fibrinolysis, Reperfusion, and the Importance of Early Treatment
The fundamental principle that benefits of coronary thrombolysis directly depend on rapid and sustained induction of infarct-related artery patency implies several considerations for optimizing results.155 Among these considerations are optimal dosage regimens with highly fibrin-selective agents, novel agents resistant to inhibitors targeted to clots, optimal antiplatelet and anticoagulant agents and regimens, and coupling later mechanical interventions to thrombolysis in patients with inadequate infarct-occluded artery luminal dimensions. No approach is more important, more powerful, more readily achieved, or more underused than the reduction of the time to treatment after onset of symptoms in patients with early, evolving acute MI. Advantages of this approach include the enhanced myocardial salvage that can be anticipated when recanalization is induced before the bulk of jeopardized ischemic myocardium is blighted,156 and the increased rapidity and extent of lysis that can be anticipated with fresh clots compared with more mature thrombi already undergoing cross-linking of fibrin123 and consequent occupancy of lysine-binding sites that otherwise serve to bind plasminogen to fibrin.
The MITI phase I pilot study157 documented an average 73-minute delay from recognition of acute MI (documented by transmission of ECG data by cellular telephone) to in-hospital treatment with coronary thrombolysis, even with highly skilled paramedical personnel. Subsequently, randomized trials were performed to identify the potential benefits of reducing this delay by implementation of prehospital coronary thrombolysis compared with in-hospital treatment.31,158,159
The importance of early treatment with fibrinolytic drugs has led to a European practice of prehospital fibrinolysis. Despite the theoretical appeal of this approach and favorable results with low mortality in several small studies, results of more recent studies of prehospital fibrinolysis without subsequent mandatory PCI compared with primary PCI are not compelling in support of this practice. Examples include the results of the Leipzig study,160 which showed superiority of primary PCI compared with administration of 50% of conventional doses of TNK combined with abciximab reflected by resolution of ST segment elevation within 90 minutes; results in the SWEDES reperfusion trial,161 in which prehospital administration of lytic agents without mandatory subsequent PCI was shown to be inferior to primary PCI despite a briefer interval between the onset of symptoms and initiation of treatment as reflected by resolution of ST segment elevation and TIMI flow grade at angiography 5 to 7 days later; and analogous results in a more recent registry study,162 in which lower 30-day and 1-year mortality was evident in patients treated with primary PCI compared with prehospital lysis without PCI regardless of whether symptoms persisted for less than or more than 2 hours before the onset of treatment, and despite the fact that prehospital lysis led to more favorable results than in-hospital lysis.
The CAPTIM study163 did not detect differences in benefit with prehospital lysis compared with primary PCI, but subsequent analysis suggested that prehospital lysis was more favorable, although not significantly (P = .058), when performed within 2 hours after onset of symptoms compared with primary PCI. The ASSENT 3-plus study,164 in which TNK was studied with unfractionated heparin compared with enoxaparin as prehospital regimens, showed better safety with unfractionated heparin with very low overall rates of the primary end points in both groups. Taken together, available data indicate that a pivotal component of favorable outcomes is the rapidity of treatment and restoration of patency of the infarct-related artery. Despite the theoretical advantage of prehospital thrombolysis consistent with this determinant, objective information in aggregate is consistent with the superiority of primary PCI to a strategy of prehospital thrombolysis in most instances. Although European practice frequently entails prehospital thrombolysis, conventional care in the United States relies more completely on PCI.
Conjunctive Therapy
The activation of circulating platelets and the blood coagulation system in patients with acute MI is a result of complex phenomena.46 Administration of plasminogen activators paradoxically contributes to these reactions. All available thrombolytic agents exhibit at least some nonspecificity, resulting in plasminemia with consequent activation of the coagulation cascade because of proteolytic cleavage of factors XII, X, and V and activation of factor X to Xa.44,45,165 Thrombin is generated in vivo, reflected by elevated concentrations in plasma of fibrinopeptide A, a cleavage product from fibrinogen elaborated by thrombin. Thrombin also activates platelets.
Suppression of coagulation and activation of platelets is necessary as conjunctive therapy to accelerate coronary recanalization, optimize its extent, and prevent reocclusion.165 We have used the term adjunctive therapy in a restricted sense to refer to measures designed to reduce myocardial injury through mechanisms independent of the mode of recanalization and without directly influencing recanalization. Examples include methods used to reduce myocardial oxygen requirements or attenuate cellular injury.
Platelet-Targeted Conjunctive Therapy
Aspirin improved survival when used in conjunction with SK in the large ISIS-2 trial.60 It is an established conjunctive agent that is usually given at an initial dose of 162 mg (chewable aspirin) as soon as possible when thrombolysis is planned, followed by daily doses of 162 to 325 mg.
Platelet activation is inhibited by aspirin through the blockade of cyclooxygenase and synthesis of thromboxane. Inhibition is incomplete, and other mechanisms can still activate platelets. A more complete inhibition can be induced by blocking the glycoprotein (GP) IIb/IIIa receptor on the platelet surface; the receptor provides the fibrinogen binding site that is required for platelet aggregation. Monoclonal antibodies directed at this receptor offer a promising approach to the blockade of platelet aggregation, which is implicated in the early thrombotic reocclusion associated with platelet-rich thrombi relatively resistant to lysis with plasminogen activators.47,166–168
Monoclonal antibodies to GP IIb/IIIa enhance lysis of platelet-rich thrombi168–170 and reduce reocclusion168,171 when administered in combination with t-PA to laboratory animals. In clinical studies, a humanized murine monoclonal antibody 7E3 Fab (abciximab) has proven promising. In the TAMI-8 pilot study,172 7E3 Fab was administered 3 hours after t-PA, heparin, and aspirin. Fewer episodes of recurrent ischemia and higher patency rates resulted. Analogous, favorable results have been obtained after PTCA.173,174 GP IIb/IIIa blockade has emerged as a valuable conjunctive measure for patients treated with PCI, but its value in association with thrombolytic agents is compromised by excess bleeding and its clearly deleterious consequences.46 In the CLARITY TIMI-28 trial,175 in which a 300-mg loading dose of clopidogrel was compared with placebo in patients treated with fibrinolytic drugs followed by mandatory angiography within 2 to 8 days and “open-label” clopidogrel subsequently, results with clopidogrel were more favorable. Significantly more patients exhibited occlusion of the infarct-related artery at angiography or death (P = .01) in the placebo-treated group (21.7%) compared with the clopidogrel group (15%). There was no difference in the incidence of mortality or the incidence of bleeding. Use of antiplatelet drugs in the adenosine diphosphate (ADP) antagonism class may be beneficial in association with administration of fibrinolytic agents.
Thrombin-Targeted Conjunctive Therapy
Unfractionated Heparin
Prevention and amelioration of the generation and activation of thrombin are important determinants of the success of coronary recanalization.176 Intravenous heparin is the most widely used conjunctive agent for this purpose. Heparin acts on thrombin indirectly, however, by complexing with antithrombin III, forming a bulky moiety that is ineffective in inhibiting clot-bound thrombin.165,177,178 Nevertheless, its antithrombin effects improve the rate of patency induced by fibrinolytic agents. Conjunctive antithrombin agents are particularly important with second-generation fibrinolytic agents because they elicit more modest elevations of circulating fibrinogen degradation products, moieties with some intrinsic anticoagulant and antiplatelet properties. Even with non–fibrin-selective agents, heparin is beneficial, however.121
The benefits of intravenous heparin (including low-molecular-weight heparin [LMWH]) in mechanistic trials are evident from the recanalization and patency rates delineated angiographically. Mortality data are also consistent with its beneficial effects.179 In the GUSTO-I angiographic study, intravenous heparin induced greater early patency than subcutaneous heparin, even with the nonselective agent SK (see Table 12-3).121
Alternatives to Unfractionated Heparin
Several studies have addressed the potential benefit of agents other than unfractionated heparin in combination with fibrinolytic drugs. In the ASSENT 3 trial,180 in which TNK plus LMWH was compared with half-dose TNK plus a abciximals an low dose unfractionated heparin and TNK+ weight-adjusted unfractionated heparin LMWH seemed to be the superior conjunctive agent with respect to the combination of efficacy and safety. Although unfractionated heparin plus a abciximals exhibited the lowest incidence of the combined end point of 30-day mortality, reinfarction, and refractory ischemia, the regimen was associated with a greater incidence of bleeding, especially in elderly patients. The ASSENT 3-plus trial,164 in which 1639 patients were studied after treatment with TNK plus enoxaparin compared with unfractionated heparin, showed superiority of enoxaparin (P = .08), but no difference in the combined end point of safety plus efficacy. There was a significant increase in intracranial hemorrhage (2.2% versus 1%; P = .047) associated with administration of the LMWH, especially in elderly patients.
In the EXTRACT TIMI-25 study,181 in which 20,479 patients were enrolled and treated with either TNK (80% of patients) or SK (20% of patients) combined with unfractionated heparin or enoxaparin, the combined end point of death or MI within 30 days was significantly lower with the LMWH enoxaparin (P < .001), with an odds ratio (OR) of 0.83 and a confidence interval (CI) of 0.77 to 0.90. There was an increased incidence of overall episodes of bleeding, but not of intracranial hemorrhage with enoxaparin, and the incidence of urgently required target vessel revascularization was lower with enoxaparin. In this study, enoxaparin dosage was reduced with respect to advanced age and other criteria, perhaps accounting in part for the favorable results.
In the OASIS VI study182 of fondaparinux, in which 12,092 patients were treated with fibrinolytic drugs or PCI combined with heparin during the procedure compared with unfractionated heparin or placebo in patients treated with SK or r-PA, results with fondaparinux were better (11.2% versus 9.7%) with respect to the incidence of the primary end point of death or MI within 30 days in patients who were not treated with PCI. Less bleeding was encountered in the patients treated with fondaparinux. The reason for administration of heparin during PCI in OASIS VI182 was that the results in the OASIS V study183 showed an unacceptable incidence of thrombus formation on guidewires without the addition of heparin to fondaparinux. Results of OASIS VI182 suggest that fondaparinux is a reasonable alternative as a conjunctive agent compared with unfractionated heparin for patients treated with thrombolytic drugs.
Hirudin
Intrinsic limitations of heparin as a pharmaceutical, particularly its dependence on antithrombin III for activity, were an impetus to the development of direct-acting antithrombins. Such agents are likely to induce more complete inhibition of thrombin. The most initially studied example is hirudin, a protein isolated originally from the medicinal leech (Hirudo medicinalis) and subsequently synthesized through recombinant technology. Hirudin binds directly to thrombin, inhibiting its catalytic and anion binding sites.178,179,184–187 In animals, hirudin exerted more favorable synergistic effects with respect to accelerating thrombolysis and sustaining patency in thrombotically occluded canine coronary arteries compared with aspirin, heparin, and a GP IIb/IIIa receptor antagonist.188
Results of three clinical trials189–191 employing hirudin in patients with acute MI or unstable angina led to a critical reappraisal of dosage and the optimal approach to balancing safety and efficacy.191 The GUSTO IIA trial189 compared intravenous heparin titrated to aPTT values of 60 to 90 seconds with intravenous hirudin at a fixed dose (0.6 mg/kg bolus and 0.2 mg/kg/hr infusion) without aPTT adjustment in patients with chest pain and ECG changes consistent with acute MI or unstable angina. This trial was designed to enroll 12,000 patients, but was terminated prematurely after 2564 patients had been studied because of an unacceptably high incidence of intracranial hemorrhage in all treatment groups. The TIMI-9A trial190 employed the same dose of intravenous hirudin and compared it with aPTT-adjusted (60 to 90 seconds) intravenous heparin combined with front-loaded t-PA or SK (the choice was made at the treating physician’s discretion) in patients with acute MI. TIMI-9A also was terminated early because of an unacceptably high incidence of intracranial and other major hemorrhage in the heparin and hirudin groups. The high incidence of complications was particularly profound in the patients treated with SK plus hirudin.
The randomized multicenter German pilot study HIT-III191 compared a different dosage and type of recombinant hirudin with heparin in combination with front-loaded t-PA (alteplase) in patients with acute MI. In all treatment groups, dosage of the antithrombin was adjusted to maintain aPTTs within a range of 2.5 to 3 times baseline values. HIT-III191 had to be terminated early because of an unacceptably high incidence of intracranial bleeding that was confined to the group treated with t-PA plus hirudin (3.4%). No intracranial bleeding occurred in the group treated with t-PA plus heparin.
The increased incidence of catastrophic bleeding encountered in these three studies might have resulted from the prolonged duration of infusion of antithrombin agents (48 to 120 hours); features of the patients studied, including advanced age (in view of the markedly low clearance of hirudin in elderly patients); high doses of the antithrombin agents associated with late bleeding as a result of adjustments based on body weight and aPTT values; and other factors that are discussed later. Regardless of the reason, the lesson to be learned is that therapeutic efficacy and safety depend on optimal dosing, as yet undefined, and its appropriate individualization. The results do not justify denigration of the value of conjunctive therapy.192 The TIMI-9B trial has been reconfigured to employ a lower dose and duration of infusion of hirudin (bolus of 0.1 mg/kg and infusion of 0.1 mg/kg/hr) and heparin (1000 U/hr without weight adjustment) plus titration of both agents to maintain aPTTs between 55 and 85 seconds.
Other direct-acting antithrombin agents, such as bivalirudin (Hirulog), have been employed in the setting of PCI with promising results.193–196 Definitive evaluations of bivalirudin in association with fibrinolytic drugs are unavailable. The HERO-2 study197 examined the use of bivalirudin or unfractionated heparin in conjunction with intravenous SK on 1-month mortality. There was no significant difference in the primary end point, although the bivalirudin group had a lower rate of reinfarction at 96 hours. Additional direct-acting antithrombins include argatroban, D-phenylalanyl-L-arginyl-chloromethylketone (P-PACK), and other chloromethylketones.198–201
Intracranial Hemorrhage and Stroke
Hemorrhage, particularly intracranial bleeding, is the major risk associated with the use of thrombolytic agents. Hemorrhage occurs through conversion of ischemic strokes to hemorrhagic ones, and by inducing a modestly higher frequency of de novo hemorrhagic strokes in patients treated with fibrinolytic drugs compared with anticoagulants alone. For patients with infarction who were treated with placebo in the early thrombolytic trials, overall early stroke incidence was approximately 1%.202 With fibrinolytic drugs, stroke incidence is 1.2% to 1.5%, with hemorrhagic stroke accounting for 0.3% to 0.7%. The risk of intracranial bleeding with t-PA is greater than with SK: 0.4% versus 0.3% in the GISSI-2 International trials142,151 and 0.7% versus 0.5% in GUSTO-I.30
Patients with infarction are at risk for emboli from carotid plaques, emboli from mural thrombi in the left ventricle, ischemic strokes that can be rendered hemorrhagic by anticoagulants or plasminogen activators, and possibly occult susceptibility to proteolysis of cerebral vessels with unsuspected pathology, such as abnormal cerebrovascular integrity as a result of an aneurysm or β-amyloid deposition (i.e., congophilic angiopathy). Patients may be prone to complications attributable to an endogenous hypercoagulable state or procoagulant effects of plasminogen activators, particularly fibrin-specific agents administered without adequate anticoagulation with intravenous heparin.202 Even before thrombolysis became part of the therapeutic armamentarium, the overall incidence of early stroke of all types accompanying acute MI ranged from 1.7% to 3.2%.203–205 In the GUSTO-I trial,30 the risk of any stroke (including intracranial bleeding) was 1.55% for patients treated with front-loaded t-PA and intravenous heparin and 1.4% for patients treated with SK and intravenous heparin.
As shown by pooling data from numerous early placebo-controlled trials of fibrinolytic agents enrolling tens of thousands of patients, the incidences of stroke were similar with and without plasminogen activators.206 The incidence of stroke overall was higher before thrombolysis was widely used than it is now.207
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree