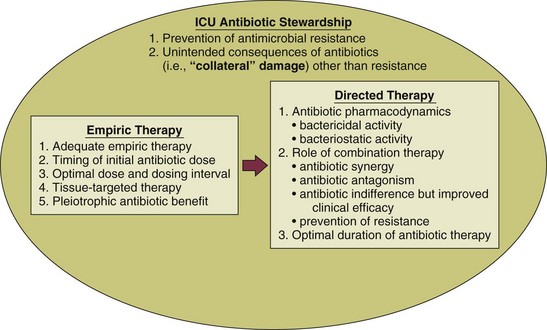
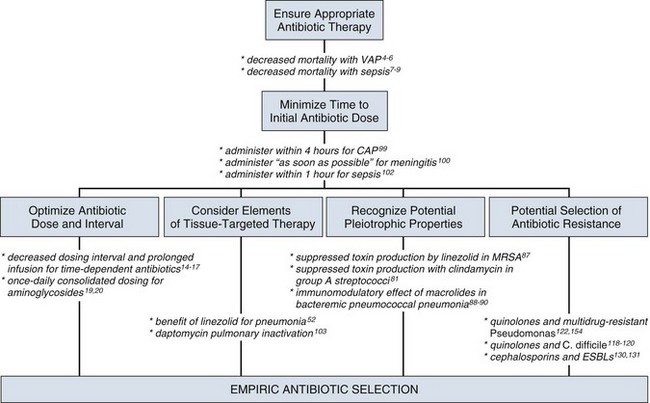
51 ADEQUACY OF INITIAL EMPIRIC ANTIBIOTIC THERAPY Penetration at the Site of Infection: Tissue-Targeted Therapy Immunomodulating Effect of Antibiotics UNINTENDED CONSEQUENCES OF ANTIBIOTIC THERAPY CLINICIAN RESPONSES TO MULTIDRUG RESISTANCE Mechanisms of Action and of Resistance The Clinical Relevance for Understanding Antibiotic Resistance MINIMIZING CLINICAL RESISTANCE In the critical care setting, the selection of optimal antibiotic therapy often entails a two-stage process: empiric therapy, followed by directed therapy once the pathogen and type of infection are clearly identified. Figure 51.1 incorporates this progression in antibiotic therapy and summarizes some principles that contribute to the goal of preserving the antibiotic armamentarium while attempting to achieve optimal clinical efficacy—the components of antibiotic stewardship. A challenge for the critical care physician is to recognize that antibiotic therapy for critically ill patients has potential ramifications for other patients in that unit over the following weeks. If antibiotic-resistant organisms or organisms with certain virulence factors are selected by a pattern of antibiotic use, those pathogens can become part of the ecology of intensive care units (ICUs) and can then be transmitted to other patients. See Figure 51.2 for clinical examples of the principles of empiric therapy. In a joint 2007 guideline paper for the development of an institutional program to enhance antimicrobial stewardship, the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) wrote on the importance of education as an essential element in any program designed to influence prescribing behavior.1 This chapter focuses on the elements of antibiotic stewardship that play a clinically relevant role in the use of antibiotics prescribed to patients in ICUs. Knowledge of these variables is essential in clinical practice and can serve as the basis for the insights necessary for an enhanced level of antibiotic prescribing. For many years clinicians felt that antibiotic therapy could be adjusted on day 2 or 3 into a clinical course, once either bacterial susceptibility was known or the clinical course of the patient had been defined, with no negative aspects of such changes. Beginning in the 1990s, several reports challenging this tenet were published regarding such infectious disease processes as sepsis and ventilator-associated pneumonia (VAP).2–8 In these reports investigators used the term inadequate to describe situations in which the organism causing an infection was not covered with antibiotic therapy as indicated by in vitro susceptibility. The published studies exhibited variability in sample size, inconclusiveness regarding whether isolated organisms were pathogens or colonizers, and lack of consistent identification of confounders that may contribute to mortality rates. On the basis of such variables, it is not possible to definitively prove that such inadequate therapy increased mortality rates. Nevertheless, the consensus of multiple studies concerned with adequate versus inadequate initial therapy in the critically ill led to the interpretation that initial inadequate therapy contributed to mortality rates.9 Shortly after the reports emphasizing the importance of initial, adequate therapy, a growing awareness of other important variables that influenced clinical outcomes in ICU patients began to emerge. In a 2005 joint guideline for management of patients with hospital-acquired pneumonia (HAP), VAP, and health care–associated pneumonia (HCAP), the American Thoracic Society (ATS) and the IDSA modified definitions for the terms used in the selection of antibiotic therapy.10 Inappropriate replaced the term inadequate; furthermore, adequate was adopted to refer to therapy that included not only the correct antibiotic based on the susceptibility of the organism but also optimal dose, correct route of administration, and use, if necessary, of combination therapy.10 As the literature regarding the treatment of infections in the ICU has evolved over the years, studies have identified several elements that influence the dose of an antibiotic that is most likely to result in the best clinical efficacy. The manner in which antibiotics kill bacteria varies among different classes of drugs, but the two pharmacodynamic categories of killing that have been best categorized are time-dependent killing and concentration-dependent killing.11 In time-dependent killing, also referred to as concentration-independent killing, maximum bacterial killing occurs when the drug concentration remains above the minimal inhibitory concentration (MIC). Examples of antibiotics that demonstrate time-dependent killing include β-lactam antibiotics (i.e., penicillins, cephalosporins, carbapenems, and monobactams) and vancomycin. Conversely, in concentration-dependent killing, maximum bacterial killing occurs when the peak drug concentration is approximately 10 times the MIC. Examples of agents with concentration-dependent killing are fluoroquinolones and aminoglycosides. The relevance of such antibiotic properties in the management of patients with serious infections has been well demonstrated. The pharmacologic properties of vancomycin have been nicely summarized, emphasizing the fact that vancomycin exhibits time-dependent killing.12 Accordingly, the length of time that concentrations of vancomycin are maintained above the pathogen’s MIC is critical to bacterial eradication, with a key variable in the treatment of pneumonia being the percentage of time that drug levels in the alveolar space exceed the MIC. As drug levels decline, organisms have the potential to regrow, increasing the chance of clinical failure. The clinical relevance of this pharmacologic principle may support the development of the practice habit of more frequent (every 6 hours) or continuous-infusion vancomycin dosing for infections like pneumonia in which vancomycin penetration to the target site may not be optimal. Because a goal with time-dependent antibiotics is to maintain levels above the MIC of the organism for as long as possible during the dosing cycle, research has explored extended-infusion dosing of several time-dependent agents, including β-lactam antibiotics and vancomycin. In a study of 194 patients with infection caused by Pseudomonas aeruginosa, piperacillin-tazobactam was administered intravenously either every 4 to 6 hours over 30 minutes or every 8 hours over 4 hours.13 The 14-day mortality rate was significantly lower among patients who received extended-infusion therapy than among patients who received intermittent-infusion therapy (12.2% versus 31.6%, respectively; P = 0.04). In another study designed to assess blood levels of antibiotic based on both dose and pattern of administration, meropenem was administered to two study groups, each with eight healthy volunteers.14 One group received 500 mg as an intravenous infusion over 30 minutes three times a day or a 250-mg loading dose followed by a 1500-mg continuous infusion over 24 hours; the second group received 1000 mg as an intravenous infusion over 30 minutes three times a day or a 500-mg loading dose followed by a 3000-mg continuous infusion over 24 hours. Investigators performed pharmacokinetic calculations and used Monte Carlo simulations for 10,000 simulated subjects. The results of the analyses of the probability of MIC attainment with the high dose were 4 mg/L with continuous infusion and 0.5 mg/L with intermittent infusion. With the low dose, results were 2 mg/L with continuous infusion and 0.25 mg/L with intermittent infusion. Such data emphasize that intermittent infusion of a low dose of a time-dependent drug may result in MICs adequate to treat relatively sensitive organisms such as Klebsiella pneumoniae but may result in less-than-optimal killing of organisms that have intrinsically higher MICs (for example, P. aeruginosa). Other reports have explored the efficacy of continuous-infusion vancomycin.15,16 A systematic review and meta-analysis suggests that extended or continuous infusion of carbapenems or piperacillin/tazobactam may be associated with lower mortality.17 The postantibiotic effect (PAE), in which microbial killing persists despite loss of detectable serum levels, complements the concentration-dependent killing of gram-negative bacilli exhibited by aminoglycosides, and these two properties may serve as the basis for once-daily aminoglycoside therapy.18 It has been suggested that giving the same total dose in larger concentration less often will result in better killing, longer PAE period, and reduced aminoglycoside toxicity that has been associated with elevated trough levels. Such pharmacologic and clinical data were the foundation for the move toward once-daily aminoglycoside dosing. In such a dosing schedule, the single doses of gentamicin or tobramycin that have been used once daily have included 5 mg/kg and 7 mg/kg of body weight. The experience with once-daily therapy using 7 mg/kg in 2184 adult patients has been reported.19 Excluded from such therapy were patients with ascites, burns involving greater than 20% total body surface area, pregnancy, end-stage renal disease requiring dialysis, and enterococcal endocarditis. The review stated that it was unnecessary to draw standard peak and trough samples and that monitoring could be completed by obtaining a single random blood sample between 6 and 14 hours after the start of an aminoglycoside infusion. The treating clinician could subsequently adjust the dosing interval in accordance with a provided nomogram. Several important observations were made in this large group of patients: (1) Despite the prolonged drug-free period, bacterial regrowth was not clinically evident; (2) no increase in either ototoxicity or nephrotoxicity was found; and (3) efficacy was promoted in a cost-effective manner.19 A meta-analysis evaluating the safety and efficacy of once-daily aminoglycosides in 1200 patients from 16 trials found no difference concerning efficacy and safety between single-dose and multiple-dose regimens.20 To achieve optimal clinical benefits while minimizing the unintended consequences of selecting resistant bacteria, clinicians must simultaneously consider multiple variables when using concentration-dependent drugs in the treatment of serious infections. In a study of lower respiratory tract infections caused by P. aeruginosa, ciprofloxacin was administered intravenously as a dose of 200 to 300 mg every 12 hours.21 Resistance emerged at a rate greater than 70% during therapy. This was similar to the 75% rate predicted by a pharmacodynamic study.22 By contrast, a randomized comparison of imipenem and ciprofloxacin for treatment of nosocomial pneumonia used a ciprofloxacin regimen of 400 mg given intravenously every 8 hours and noted emergence of resistance during therapy in 33% of cases in which Pseudomonas was the causative pathogen.23 This rate was similar to the 38% predicted by the pharmacodynamic study previously cited.22 These data emphasize the importance of considering not only the dosing interval but also the dose of antibiotic administered when using concentration-dependent antibiotics to treat infections in the critically ill. In addition to dosing interval and initial dose, antibiotic clearance plays an integral role in adequacy of antibiotic therapy. Although it is routinely accepted that reductions in antibiotic dosing are appropriate in advancing acute kidney injury,24 clinicians may fail to escalate drug administration in the increasingly reported clinical phenomenon of augmented renal clearance (ARC).25 ARC is defined as the enhanced renal elimination of circulating solutes due to an increased creatinine clearance (CrCl > 130 mL/minute/1.73 m2). Prevalence of ARC is higher in burn,26 major trauma,27 traumatic brain injury,28 and febrile neutropenic29 ICU patients; however, it is difficult to traditionally recognize as derived estimates of glomerular filtration, such as the modification of diet in renal disease (MDRD) equation and Cockcroft-Gault (CG) formula are less accurate than direct measurement.30 In 48 ICU patients with measured CrCl, Udy and colleagues demonstrated that CrCl values 130 mL/minute/1.73 m2 or greater were associated with initial trough concentrations of β-lactams less than MIC in 82% (P < 0.001). Moreover, CrCl remained a significant predictor of subtherapeutic concentrations after multivariate analysis.31 ARC has importance in vancomycin dosing as well. Baptista and associates performed a prospective, single-center, observational cohort study of 93 consecutive septic patients in the ICU treated with vancomycin by continuous infusion after a weight-based loading dose. Investigators compared patients with and without ARC. A statistically significant correlation between subtherapeutic vancomycin serum concentration and ARC was observed in the first 3 days of treatment.32 An interaction between renal clearance and hypoalbuminemia also plays a prominent role in pharmacokinetics. In fact, Ulldemolins and colleagues demonstrated that in critically ill patients with hypoalbuminemia, unbound flucloxacillin concentrations fell below concentrations necessary for treatment of methicillin-susceptible Staphylococcus aureus (MSSA) 4 hours after standard bolus infusions.33 The combination of increased volume of distribution due to hypoalbuminemia combined with ARC presents a daily situation in which lower achieved antibacterial exposures could result in subtherapeutic dosing, particularly for time-dependent antibiotics.34 Because accurate and timely drug exposure is necessary for optimal clinical benefit,3 ARC and associated factors like hypoalbuminemia must be identified to prevent subtherapeutic pharmacologic treatment. The importance of antibiotic pharmacokinetic properties has recently received heightened attention for the treatment of pneumonia caused by multidrug-resistant gram-positive cocci. Indeed, pulmonary pharmacokinetics underlie the findings that the use of linezolid for pneumonia caused by methicillin-resistant S. aureus (MRSA), when compared with vancomycin in traditional regimens, results in improved clinical outcomes.35–38 Understanding the relevance of pharmacokinetic properties will help the critical care physician navigate through the recent published literature for these infections. Pulmonary pharmacokinetics specifically address the tissue penetration and distribution of antibiotics within the lung.39 Early studies investigating antibiotic penetration in pulmonary infections occurring in the ICU were related to aminoglycosides and fluoroquinolones. Although aminoglycoside levels in the interstitium of the lung are acceptable, levels in pulmonary secretions reach a mean of only about 20% of the concomitant serum level.40 By contrast, the concentrations of quinolones in lung tissue significantly exceed the concomitant serum concentrations, and levels in bronchial secretions also have been reported to exceed those in serum.41 Despite the fact that quinolones have better penetration into the lung and less potential for nephrotoxicity than aminoglycosides, available data show a trend toward improved survival in patients with VAP treated with an aminoglycoside-containing, but not with a quinolone-containing, combination.42 A concern with fluoroquinolones in combination therapy directed against gram-negative organisms is the selection of resistance, particularly in organisms such as P. aeruginosa, in which the resultant resistance may be to multiple classes of antibiotics.43 Because of the coexistent potential for clinical efficacy and nephrotoxicity with aminoglycosides, some investigators have suggested, on the basis of clinical trials, discontinuation of the aminoglycoside after 5 days if the patient is improving.44 Existing pharmacokinetic evidence reveals the extremely poor lung tissue penetration of vancomycin. Cruciani and associates investigated vancomycin pharmacokinetics in 30 human lung tissue sections after administration of a 1-g dose over 1 hour.45 A comparison of serum-to-tissue concentration over the dosing interval was used to generate a graph allowing determination of a concentration ratio. Overall, the serum-to-lung tissue concentration ratio was determined to be 21%. Not surprisingly, investigation has confirmed even poorer penetration into epithelial lining fluid.46 These data raise concern that the traditional dosing regimens of vancomycin (1 g given intravenously every 12 hours) and low target serum trough concentrations (5 to 10 µg/mL) will generate lung tissue concentrations below the MIC for S. aureus. The issues surrounding suboptimal vancomycin dosing are reflected in the 2005 published ATS/IDSA guidelines for treatment for adults with HAP, VAP, and HCAP, in which it was noted that retrospective pharmacokinetic modeling suggested that the vancomycin failures may be related to inadequate dosing. Because of these concerns the authors recommend that trough levels for vancomycin should be 15 to 20 µg/mL.10 This recommendation of intensified vancomycin dosing is supported in a joint vancomycin clinical guideline for treating all complicated infectious caused by MRSA.47 In contrast with the poor pulmonary pharmacokinetic properties of vancomycin, several studies of linezolid confirm excellent lung penetration in healthy volunteer subjects and with in vitro modeling.48–50 Boselli and coworkers investigated the steady-state plasma pharmacokinetic variables and epithelial lining fluid concentrations of linezolid administered to critically ill patients with VAP.51 Epithelial lining fluid concentrations of linezolid approximated 100% of corresponding plasma values, with drug concentrations that exceeded the susceptibility breakpoint (4 mg/mL) for S. aureus throughout the greater part of the dosing interval. The principle of antibiotic pharmacokinetics has supported the conclusion that linezolid is superior to vancomycin in traditional dosing regimens for MRSA pneumonia based on retrospective analysis of the two multinational, double-blind, randomized studies published to date.38 A subsequent randomized, controlled clinical trial of linezolid versus vancomycin in the treatment of nosocomial pneumonia favored linezolid; furthermore, a subgroup analysis of this investigation did not suggest a benefit in those patients with vancomycin troughs of 15 mg/L or more on day 3.52 In light of the difficulty in achieving adequate drug levels in pulmonary tissue, many clinicians consider the use of topical, aerosolized antibiotics to deliver drug directly to the site of infection. The agents most commonly employed in this manner are tobramycin and colistin.53 Most of our experience with, and evidence supporting, nebulized tobramycin originates from small studies and meta-analyses assessing the utility of these agents in the management of chronic respiratory disease such as cystic fibrosis, not in the treatment of acute infection.54 Although the risk of toxicity appears lower with the administration of nebulized tobramycin, certain populations, such as those with renal insufficiency, may be at risk.55 Because of the increasing prevalence of multidrug-resistant gram-negative organisms such as Acinetobacter baumannii and P. aeruginosa, clinicians have employed colistimethate via intravenous infusion56 or aerosolization57,58 as therapy for VAP and HAP. Intravenous colistimethate appears to be safe (with a risk of nephrotoxicity similar to that for intravenous aminoglycosides) and effective, though there are few randomized, controlled trials from which to draw firm conclusions; furthermore, the optimal dosing strategy is unclear.59 Although the aerosolized route of colistimethate administration appears to be safe,60 its efficacy has not been established. One of the most frequently cited justifications for combination therapy is for the achievement of synergy, in which antimicrobial combinations are more effective than single agents. The best-recognized example of synergistic antimicrobial therapy is in treatment of enterococcal endocarditis, in which treatment with penicillin or ampicillin alone has been associated with a high rate of relapse when compared with therapy with penicillin or ampicillin in combination with streptomycin or gentamicin.61,62 Discussions of combination therapy have raised the question of whether the use of multiple drugs in the treatment of an infection may result in improved clinical outcomes. For example, in the treatment of bacteremia with S. aureus, some investigators have used a semisynthetic penicillinase-resistant penicillin (e.g., nafcillin or oxacillin) in combination with a brief course (3 to 5 days) of an aminoglycoside, based on data showing more rapid clearing of bacteremia.63 Data from this trial did not show a decrease in mortality rates in the study population of nonaddicts with primarily left-sided endocarditis caused by S. aureus when compared with those patients who received nafcillin alone. For P. aeruginosa the mechanism of synergy between antipseudomonal penicillins and aminoglycosides is similar to that of enterococci.64 Despite this microbiologic observation, the presence or absence of synergy seemed less important in a different trial assessing outcomes in patients with P. aeruginosa bacteremia; rather, this investigation explored combination therapy given in an attempt to prevent the emergence of resistance.65 In contrast with the beneficial effect of synergy, a combination regimen may result in the detrimental effect of antagonism. The classic example of such an effect was with the treatment of pneumococcal meningitis in the 1950s, in which the fatality rate among patients who received penicillin alone was 21%, in contrast with 79% among those who received both penicillin (a bactericidal agent) plus chlortetracycline (a bacteriostatic agent).66 The clinical importance of antagonism has increased in the era of community-associated methicillin-resistant S. aureus (CA-MRSA). Published evidence supports the fact that MRSA is an independent predictor of mortality rate, ICU length of stay, and overall cost of care.67–70 Because a limited number of therapeutic options exist for the treatment of severe, invasive MRSA infections, effort has been made to identify the in vitro activity of antibiotic combinations that may have clinical applicability. Although the intent of using antibiotic combinations is to achieve additive or synergistic effects, the use of combinations may have the unintended effect of antagonism. A study using 10 different strains of S. aureus found an overall pattern of antibiotic indifference (no combination effect) in combinations of linezolid with fusidic acid, rifampin, or gentamicin. On the other hand, the combination of linezolid with ciprofloxacin or vancomycin resulted in slight antagonism and reduced bactericidal effect when linezolid was combined with ciprofloxacin and vancomycin against the same strains of staphylococci.71 A subsequent study using the checkerboard broth microdilution method tested linezolid in combination with 28 different antimicrobial agents—including vancomycin and several fluoroquinolones—and demonstrated no antagonistic effect.72 Sahuquillo and colleagues reproduced the effect of antibiotic indifference with vancomycin, but they noted antagonism with levofloxacin in two of the five S. aureus isolates tested.73 Another investigation used the rabbit model of aortic valve endocarditis to evaluate 5-day treatment regimens of linezolid alone, vancomycin alone, and linezolid in combination with vancomycin in 40 rabbits infected with an MRSA strain.74 Those treated with vancomycin alone demonstrated greater mean reductions in valvular vegetation bacterial counts than those in the other treatment groups (P = 0.05). Vancomycin also sterilized aortic valve vegetations in three of eight rabbits; by contrast, none of the rabbits treated with linezolid had sterile aortic valve vegetations. A noteworthy finding in this study was that the treatment regimen of linezolid plus vancomycin lowered the peak linezolid levels in serum to below those obtained with regimens with linezolid alone. Even though in vitro synergy testing revealed additive or indifferent activity between the two drugs, the rabbit model revealed in vivo antagonism. A potential explanation offered by the investigators for the observed antagonism between vancomycin and linezolid was the effect of combining a bacteriostatic (linezolid) with a bactericidal drug (vancomycin). The observed reduction in peak linezolid levels in serum with the combination of the two drugs suggests a role for additional mechanisms in the interaction between the two antibiotics. Unfortunately, the clinical significance of these findings is not yet known. In the absence of definitive data on optimal management of S. aureus infections in seriously ill patients, a pattern has emerged in some health care systems to prescribe combination therapy for this pathogen. The overall assessment of the data is that antibiotic indifference appears to best characterize the drug interaction profile of linezolid with vancomycin. Because some data, albeit unsubstantiated in controlled clinical trials, seem to cast doubt on the advisability of the combination of linezolid with vancomycin71 and the fluoroquinolones,73 it is important for the intensivist to be aware of the potential consequences of doing so. In short, the role of combination therapy in the treatment of serious MRSA infection, except in a few specific instances, is unclear.75 A common question in clinical medicine is whether combination therapy will result in increased efficacy against a pathogen via a mechanism other than synergy. In an attempt to find a more definitive answer to this question, a meta-analysis of 64 trials with 7586 patients comparing β-lactam monotherapy versus β-lactam plus an aminoglycoside in immunocompetent patients with sepsis was conducted.76 This report did not identify a statistically significant advantage of combination therapy among the 1835 patients with gram-negative infections for whom the data were analyzed. In contrast with the results in the previously cited study,65 no improved survival was observed for the 426 patients who had infection caused by P. aeruginosa. An additional finding was that the rates of development of resistance did not differ in the two treatment groups. Nephrotoxicity, however, developed significantly more often in those patients who received combination therapy. Clinicians frequently use combination therapy for P. aeruginosa in an attempt to prevent the emergence of resistance. Despite the importance of this subject, no definitive data are available to prove that combination therapy will prevent the emergence of Pseudomonas resistance;77 however, results of clinical trials23 and concern about this possibility based on limited data have been the basis for such use of combination therapy. A meta-analysis of eight randomized controlled clinical trials compared β-lactam monotherapy and β-lactam plus aminoglycoside combination therapy to assess if combination therapy may decrease the risk of the emergence of resistance.78 Among initially antimicrobial-susceptible isolates in this analysis, combination therapy did not impact the development of antimicrobial resistance. Furthermore, in the meta-analysis of 64 trials comparing β-lactam monotherapy and β-lactam plus aminoglycoside combination therapy in immunocompetent patients with sepsis, there was no difference in the rate of development of resistance.76 Even though issues of synergy and reduction in the emergence of resistance frequently are invoked in discussions of combination therapy, the relevant data do not prove consistent benefits. In patients in the intensive care setting, an important advantage of combination therapy is that it provides the clinician with broader antibacterial coverage for potentially multidrug-resistant microorganisms.79 Additionally, because inappropriate initial therapy may result in increased mortality rate, a combination of antibiotics has the potential benefit of providing coverage against a pathogen that may not be the most likely on a statistical basis but is a reasonable consideration in life-threatening clinical settings confronting the critical care physician. The use of antibiotic combinations for empiric therapy increases the probability that at least one of the agents will have activity against the causative organism. Streptococcal toxic shock syndrome is a clinical infection in which bacteria produce exotoxins that act as host superantigens, precipitating shock, multiple organ failure, and death. Although Streptococcus pyogenes demonstrates exquisite in vitro sensitivity to penicillin, experimental studies of infection by this pathogen have demonstrated reduced efficacy against organisms in the stationary phase of bacterial growth. This phenomenon has been termed the Eagle effect, whereby high organism population density and slow organism division make treatment with an antibiotic dependent on cell wall synthesis ineffective.80 Some cite the Eagle effect as a justification for use of the bacteriostatic antibiotic clindamycin in the treatment of toxic shock syndrome. In addition, clindamycin is an antibiotic that inhibits bacterial protein synthesis, and this pharmacodynamic property is independent of the stage of bacterial growth.81 Clindamycin inhibits bacterial exotoxin production, facilitates phagocytosis of S. pyogenes by inhibiting M protein synthesis, and suppresses the production of penicillin-binding proteins (PBPs). Furthermore, evidence exists that demonstrates clindamycin has immunomodulatory effects, suppressing monocyte synthesis of tumor necrosis factor-α (TNF-α).82,83 All of these pleiotropic qualities have resulted in the recommendation for clindamycin use in necrotizing skin or soft tissue infections and toxic shock syndrome caused by S. pyogenes.81 The dramatic increase in the worldwide incidence of highly virulent, community-acquired infection with CA-MRSA has resulted in increasing reports of necrotizing skin and soft tissue infections as well as necrotizing pneumonia confronting the critical care physician.84–86 CA-MRSA virulence has been attributed to expression of several virulence factors: α-hemolysins (AH), toxic shock syndrome toxin-1 (TSST-1), staphylococcal enterotoxin B, and Panton-Valentine leukocidin (PVL). The association of staphylococcal virulence with the current CA-MRSA epidemic prompted Stevens and colleagues to investigate the impact of antibiotics on the expression of virulence-associated exotoxin genes.87 These investigators were able to demonstrate markedly suppressed in vitro production of staphylococcal toxin genes by clindamycin and linezolid such that no PVL production was noted up to 12 hours after antibiotic administration. Of interest, subinhibitory concentrations of the cell wall–active agent nafcillin were found to increase toxin production. These findings led the investigators to conclude that the inhibition of protein synthesis is an important consideration in the selection of antimicrobial agents for treatment of serious infections caused by toxin-producing gram-positive cocci.87 A growing body of evidence exists to support the benefit of macrolide therapy for bacteremic pneumonia caused by Streptococcus pneumoniae.88–96 Although multiple explanations have been proposed, efficacy appears to extend beyond the drug’s spectrum of antimicrobial activity. The macrolide class of antibiotics exerts a broad range of immunomodulatory effects, including suppression of harmful interleukin host responses and inhibition of neutrophil oxidant burst and degranulation.88–90 These pleiotropic effects have received an increasing focus of attention and further illustrate how immune modulation influences recommendations for therapy in the intensive care setting. The impact of the timing of antibiotic therapy has been addressed in several ways with regard to patients in the ICU. In an analysis based on 107 consecutive patients receiving mechanical ventilation and antibiotic treatment for VAP, Iregui and colleagues noted that 30.8% (33 of 107) received antibiotic treatment that was delayed for 24 hours or more after initially meeting diagnostic criteria for VAP; these patients were classified as having initially delayed appropriate antibiotic therapy (IDAAT).97 Two major variables were identified in these patients with IDAAT: (1) a delay in writing an antibiotic order (in 75.8% of the cases); and (2) the presence of a bacterial species resistant to the initially prescribed antibiotic regimen (in 18.2%). The investigators found that hospital mortality rate was 69.7% for the patients with IDAAT, in contrast with only 28.4% for the patients without IDAAT (P < 0.01). An earlier study noted that, in patients with VAP, modification of the antibiotic regimen to cover pathogens based on the susceptibility report did not eliminate the increased mortality rate associated with inadequate empiric therapy.5 Acknowledgment of this finding was the basis for the statement that secondary modifications of an initially failing antibiotic regimen do not substantially improve the outcome for critically ill patients.98 These results challenge the clinician to promptly order antibiotics that cover the involved pathogens even before culture results are available. The importance of antibiotic timing in patients with community-acquired pneumonia was assessed in a retrospective cohort study of pneumonia in 18,209 Medicare patients.99 In this trial, conducted in a random sample of inpatients 65 years of age or older with community-acquired pneumonia who had not received antibiotics as outpatients, the influence on clinical outcome was assessed for use of antibiotics prescribed according to standard guidelines published at the time of the analysis and not identification of pathogens isolated from the patients. Of the patients who received antibiotics within 4 hours of hospital arrival, 83.2% were prescribed a guideline-recommended regimen, in contrast with 71.8% of the patients who received antibiotics after 4 hours of arrival. The results of this analysis found an association between the administration of antibiotics within 4 hours of hospital arrival and a decrease in mortality rate and length of stay. The investigators postulated that antibiotics may interrupt or minimize the effects of the acute lung injury process that occurs as part of the systemic inflammatory response in patients with bacterial pneumonia. The IDSA practice guidelines for management of adult patients with meningitis note the lack of prospective clinical data on the relationship of the timing of antimicrobial administration of antimicrobial agents to clinical outcome in patients with bacterial meningitis.100 Data from a retrospective cohort study of 269 adult patients with community-acquired bacterial meningitis provide some insights into the timing of antibiotic therapy in the absence of definitive recommendations.101 Using these and other data, the IDSA acknowledged that evidence for definitive recommendations is inadequate and concluded that a reasonable assumption is to administer treatment for bacterial meningitis before the infection advances to a high level of clinical severity.100 Referring to meningitis as a “neurologic emergency,” the guideline recommended that appropriate therapy for meningitis be initiated as soon as possible after the diagnosis is considered to be likely.100 In support of the importance of prompt timing, this document also noted the potential in certain patients for administration of antibiotics before hospital admission if the patient initially presents outside the hospital. For the critical care clinician, sepsis is a clinical entity in which adequate antibiotic therapy has been associated with improved clinical outcomes.2,3,7,8,79 In recognition of the importance of prompt therapy in influencing clinical outcomes in patients with sepsis, the 2008 Surviving Sepsis Campaign guidelines offered a specific recommendation regarding the timing of antimicrobial therapy for the septic patient: “Intravenous antibiotic therapy should be started as early as possible and within the first hour of recognition of septic shock and severe sepsis without septic shock. Appropriate cultures should be obtained before initiating antibiotic therapy but should not prevent prompt administration of antimicrobial therapy.”102 As the focus of antibiotic research has expanded beyond the characterization of in vitro properties for a particular agent, the importance of antibiotic performance at the in vivo target tissue level is becoming increasingly recognized. It was with the 2003 introduction of a novel antibiotic, daptomycin, for treatment of infections caused by resistant gram-positive cocci that the relevance of “organ-specific deactivation” initially was described.103 Daptomycin is an intravenous cyclic lipopeptide with rapid, concentration-dependent killing and bactericidal activity against a broad spectrum of gram-positive cocci.104–106 It demonstrates a unique mechanism of action, with calcium-dependent insertion into the phospholipid bacterial cell membrane. This results in cell depolarization via potassium efflux, causing disruption of DNA, RNA, and protein synthesis.107 As a result of two multicenter, randomized controlled trials comparing it with penicillinase-resistant penicillin or vancomycin,108 daptomycin, at a daily dose of 4 mg/kg (for patients with CrCl greater than 30 mL/minute), received U.S. Food and Drug Administration (FDA) approval for the treatment of complicated skin and soft tissue infections. A subsequent randomized, open-label trial investigated use of daptomycin in S. aureus bacteremia and endocarditis.109 This trial prompted FDA approval of daptomycin for treatment of S. aureus bacteremia, including right-sided endocarditis, at a daily intravenous dose of 6 mg/kg. Phase 3 clinical trials also were conducted for the treatment of community-acquired pneumonia in hospitalized patients. Despite daptomycin’s potent in vitro bactericidal activity against S. pneumoniae, clinical outcomes were disappointing and inferior to those with the comparator, ceftriaxone. Although daptomycin is known to exhibit poor penetration into epithelial lining fluid, the reason for treatment failure was not fully elucidated until Silverman and colleagues described a unique organ-specific inactivation process.103 Daptomycin’s inactivation was linked to its mechanism of bactericidal action: calcium-dependent membrane lipid binding.103,107,110,111 Using a mouse model, investigators demonstrated drug sequestration and inactivation by binding to phospholipid vesicles that are found in pulmonary surfactant.103 For this reason daptomycin is not considered an appropriate therapeutic agent for treatment of pneumonia, largely because of the presence of surfactant at the target tissue. This phenomenon appears to be unique to daptomycin103 but raises the question of unrecognized organ-specific inactivation for other classes of antibiotics and other target organs. Traditional teaching about antibiotics focused on three classic parameters: efficacy, safety, and cost-effectiveness. With respect to safety, the major considerations were allergic reactions and adverse effects. Within the pandemic of antibiotic resistance, an important new safety issue should be added: unintended consequences of antibiotic therapy. An insightful report termed such unintended consequences the “collateral damage” of antibiotics.112 In this report, collateral damage referred to the ecologic adverse effect of selecting drug-resistant organisms and the unwanted development of colonization or infection with multidrug-resistant organisms. Some important new considerations recently described in the literature, however, may influence initial antibiotic selection for serious infections in the ICU.
Principles Governing Antimicrobial Therapy in the Intensive Care Unit
Adequacy of Initial Empiric Antibiotic Therapy
Optimal Dose
Penetration at the Site of Infection: Tissue-Targeted Therapy
Role of Combination Therapy
Synergy
Enhanced Efficacy Against a Pathogen
Prevention of the Emergence of Resistance
Increased Opportunity for Achieving Appropriate Therapy
Immunomodulating Effect of Antibiotics
Timing
Special Pharmacologic Properties
Unintended Consequences of Antibiotic Therapy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Anesthesia Key
Fastest Anesthesia & Intensive Care & Emergency Medicine Insight Engine