Chapter 13 Preoxygenation
I Historical Perspective
In 1948, Fowler and Comroe demonstrated that inhalation of 100% oxygen (O2) resulted in a very rapid increase of arterial oxyhemoglobin saturation (SaO2) to between 98% and 99%, but that attainment of the last 1% to 2% was a much slower process.1 They also observed that the rate of increase was attenuated in patients with pulmonary emphysema or pulmonary artherosclerosis.1 In 1955, Hamilton and Eastwood showed that “denitrogenation” was 95% complete within 2 to 3 minutes in subjects breathing normally from a circle anesthesia system with 5 L/min O2.2 Dillon and Darsi, in the same year, observed significant arterial oxyhemoglobin desaturation during apnea after anesthetic induction with sodium thiopental and recommended that induction of anesthesia and endoscopy should be preceded by O2 administration for 5 minutes.3 Six years later, Heller and Watson found that 3 to 4 minutes of O2 breathing was necessary in patients before anesthetic induction, whereas adequate oxygenation could be accomplished with the use of manual ventilation in 30 seconds.4 With the introduction of the rapid-sequence induction and intubation (RSI) technique in the 1950s in patients at risk for aspiration of gastric contents, preoxygenation became a component of the technique.5,6 Preoxygenation was emphasized by Sellick when he introduced cricoid pressure into clinical practice in 1961,7 and it was also recommended before RSI in pediatric patients in the 1970s.8
Preoxygenation before anesthetic induction and intubation became a widely accepted maneuver designed to increase O2 reserves and thereby delay the onset of arterial oxyhemoglobin desaturation during apnea. Various techniques and regimens have been advocated to ensure adequate preoxygenation. For many years, tidal volume breathing (TVB) of O2 for 3 to 5 minutes has been commonly practiced.2,3 Gold and colleagues challenged the need for 3 minutes of TVB by demonstrating that 4 deep breaths within 0.5 minutes (4 DB/0.5 min) and TVB for 5 minutes using a semiclosed circle absorber system were equally effective in increasing arterial oxygen tension (PaO2).9 Although some investigators corroborated their findings,10,11 others showed that TVB for 3 minutes provided better preoxygenation and longer protection against hypoxemia during apnea than 4 DB/0.5 min.12–15 Later investigations suggested that the use of extended deep breathing (8, 12, and 16 deep breaths in 1.0, 1.5, and 2.0 minutes, respectively) could produce maximal preoxygenation comparable to that achieved with TVB for 3 minutes and could also delay the onset of apnea-induced oxyhemoglobin desaturation.16,17
Regardless of the technique used, preoxygenation has become an integral component of the RSI technique, and it is particularly important if manual ventilation is not desirable, if difficulty with ventilation or endotracheal intubation is anticipated, and in patients with oxygen transport limitations. Because the “cannot intubate, cannot ventilate” (CICV) situation is largely unpredictable, the desirability of maximal preoxygenation is theoretically present for all patients.18
The original American Society of Anesthesiologists’ (ASA) difficult airway (DA) algorithm made no mention of preoxygenation. In an updated report by the ASA Task Force on Management of the DA (2003), the topic of “facemask preoxygenation before initiating management of the difficult airway” was added.19 “Routine” preoxygenation has become a new “minimum standard” of care, not only during induction of anesthesia, but also during emergence from anesthesia and tracheal extubation.20–22
II Body Oxygen Stores
Oxygen is carried in the blood in two forms: the greater portion is in reversible chemical combination with hemoglobin (Hb), and the smaller part is dissolved in plasma.23 The ability to carry large amounts of O2 in Hb is important, because without it, the amount carried in the plasma would be so small that the cardiac output would need to be increased more than 20 times to yield an adequate O2 flux.23 The amount of chemically bound O2 is directly related to the concentration of Hb and how saturated the Hb is with O2. Arterial O2 content (Cao2) can be calculated from the following equation:
1.36 = estimated mass volume of O2 that can be bound by 1 g of normal Hb
SaO2 = arterial oxyhemoglobin saturation (when fully saturated, SaO2 = 100%)
PaO2 = arterial partial pressure of oxygen
Hb uptake and release of O2 are regulated by a pattern demonstrated by the familiar oxyhemoglobin dissociation curve, which is a plot of SaO2 as a function of PaO2. The sigmoid shape of the curve reflects the fact that the four binding sites on a given Hb molecule interact with each other.23 When the first site has bound a molecule of O2, the binding of the next site is facilitated, and so forth. The result is a curve that is steep up to a PO2 of 60 mm Hg and becomes more shallow thereafter, approaching 100% saturation asymptotically. At a PO2 of 100 mm Hg, the normal arterial value, 97% of the hemes have bound O2; at 40 mm Hg, a typical value for (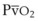 ) in a resting person, the saturation declines to about 75%. The shape of the oxyhemoglobin dissociation curve has important physiologic implications. The flatness of the curve above a PO2 of 80 mm Hg ensures a relatively constant SaO2 despite wide variations in alveolar O2 pressure. The steep portion of the curve between 20 to 60 mm Hg permits unloading of O2 from Hb at relatively high PO2 values, which favors the delivery of large amounts of O2 into the tissues by diffusion.
) in a resting person, the saturation declines to about 75%. The shape of the oxyhemoglobin dissociation curve has important physiologic implications. The flatness of the curve above a PO2 of 80 mm Hg ensures a relatively constant SaO2 despite wide variations in alveolar O2 pressure. The steep portion of the curve between 20 to 60 mm Hg permits unloading of O2 from Hb at relatively high PO2 values, which favors the delivery of large amounts of O2 into the tissues by diffusion.
The O2-binding properties of Hb are influenced by a number of factors, including pH, PCO2, and temperature.23 These factors cause shifts of the oxyhemoglobin dissociation curve to the right or left without changing the slope of the curve. For example, an increase in temperature or a decrease in pH, such as may occur in active tissues, decreases the affinity of Hb for O2 and shifts the oxyhemoglobin dissociation curve to the right. As a result, a higher PO2 is required to achieve a given saturation, which facilitates unloading of O2 at the tissue. To quantify the extent of a shift of the oxyhemoglobin dissociation curve, the so-called P50 is used—that is, the PO2 required for 50% saturation. The P50 of normal adult Hb at 37° C and normal pH and PCO2 is 26 to 27 mm Hg.
Despite its great importance, O2 is a very difficult gas to store in a biologic system. In subjects breathing air, O2 stores are small (Table 13-1).23,24 The relatively steep oxyhemoglobin dissociation curve and the small O2 stores imply that factors affecting PaO2 produce their full effects very quickly. This is in contrast to CO2, for which the large size of the stores buffers the body against rapid changes. Therefore, in a subject breathing air, a pulse oximeter probably gives an earlier indication of hypoventilation than does CO2 measurement. In contrast, in a subject breathing a high fraction of inspired O2 (FIO2), CO2 measurement gives an earlier indication of hypoventilation.23
Table 13-1 Body O2 Stores (in mL) during Room Air and 100% O2 Breathing
| Storage Site | Room Air | 100% O2 |
|---|---|---|
| In the lungs (FRC) | 450 | 3000 |
| In the blood | 850 | 950 |
| Dissolved in tissue fluids | 50 | 100 |
| In combination with myoglobin | 200? | 200 |
| Total | 1550 | 4250 |
FRC, Functional residual capacity; O2, oxygen.
From Nunn JF, editor: Nunn’s applied respiratory physiology, ed 4, Oxford, 1993, Butterworth-Heinemann, p 288.
The amounts of body O2 in the various storage sites of a person breathing air are increased with breathing 100% of O2 (Fig. 13-1; see also Table 13-1).23,24 The maximal increase of O2 stores occurs in the functional residual capacity (FRC). Storage of O2 in the tissue is rather difficult to assess, but assuming that Henry’s law applies and the partition coefficient for gases approximates the gas-water coefficients, breathing O2 for 3 minutes significantly increases tissue O2 stores.24
III Physiology of Apnea and Apneic Mass-Movement Oxygenation
During apnea, the total body O2 consumption (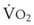 ) remains fairly constant at about 250 mL/min. Consequently, the alveolar O2 concentration (PAO2) decreases rapidly as a result of the depletion of the diminishing O2 stores in the lungs. If the airway becomes obstructed, O2 removal will generate a substantial and immediate negative pressure, contributing to a further decrease of PaO2. Although the PaO2 falls in direct relation to PAO2, SaO2 remains greater than 90% as long as the Hb can be reoxygenated in the lungs.25–28 SaO2 starts to decrease only after the lung O2 stores are depleted and the PaO2 is lower than 60 mm Hg. It is for this reason that oximetry is not the best “physiologic means,” compared with PaO2, for predicting the onset of hypoxemia. However, because it detects decreases in SaO2 before other clinical signs, oximetry is an invaluable clinical monitor that adds to the safety of anesthetic management.27 Critical oxyhemoglobin desaturation may be defined as SaO2 less than or equal to 80%; for patients with SaO2 less than 80%, the range in the rate of decrease is 20% to 40% per minute during apnea.29
) remains fairly constant at about 250 mL/min. Consequently, the alveolar O2 concentration (PAO2) decreases rapidly as a result of the depletion of the diminishing O2 stores in the lungs. If the airway becomes obstructed, O2 removal will generate a substantial and immediate negative pressure, contributing to a further decrease of PaO2. Although the PaO2 falls in direct relation to PAO2, SaO2 remains greater than 90% as long as the Hb can be reoxygenated in the lungs.25–28 SaO2 starts to decrease only after the lung O2 stores are depleted and the PaO2 is lower than 60 mm Hg. It is for this reason that oximetry is not the best “physiologic means,” compared with PaO2, for predicting the onset of hypoxemia. However, because it detects decreases in SaO2 before other clinical signs, oximetry is an invaluable clinical monitor that adds to the safety of anesthetic management.27 Critical oxyhemoglobin desaturation may be defined as SaO2 less than or equal to 80%; for patients with SaO2 less than 80%, the range in the rate of decrease is 20% to 40% per minute during apnea.29
Preoxygenation followed by O2 insufflation during subsequent apnea maintains SaO2 by apneic diffusion oxygenation.25,26 In the apneic adult, the 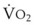 averages 230 mL/min, whereas the output of CO2 to the alveoli is limited to about 21 mL/min and the remaining CO2 production (approximately 90%) is buffered within the body tissues. The lung volume initially decreases by the net gas exchange ratio of 209 mL/min. Therefore, a pressure gradient is created between the upper airway and the alveoli, and if the airway is patent, this results in a mass movement of O2 down the trachea into the alveoli. Conversely, CO2 is not exhaled because of this mass movement of O2 down the trachea, and the alveolar CO2 concentration (PACO2) shows an initial rise of about 8 to 16 mm Hg during the first minute and a subsequent fairly linear rise of about 3 mm Hg/min.30
averages 230 mL/min, whereas the output of CO2 to the alveoli is limited to about 21 mL/min and the remaining CO2 production (approximately 90%) is buffered within the body tissues. The lung volume initially decreases by the net gas exchange ratio of 209 mL/min. Therefore, a pressure gradient is created between the upper airway and the alveoli, and if the airway is patent, this results in a mass movement of O2 down the trachea into the alveoli. Conversely, CO2 is not exhaled because of this mass movement of O2 down the trachea, and the alveolar CO2 concentration (PACO2) shows an initial rise of about 8 to 16 mm Hg during the first minute and a subsequent fairly linear rise of about 3 mm Hg/min.30
Fraioli and colleagues emphasized the importance of the ratio of FRC to body weight during apneic diffusion and demonstrated that patients with a low FRC/body weight ratio could not tolerate apnea for more than 4 minutes, whereas those with a high FRC/body weight ratio (>53.3 ± 7 mL/kg) maintained PaO2 at 90% of the control value.25 Some studies demonstrated that with a patent airway and an FIO2 of 1, SaO2 can be maintained at greater than 90% for up to 100 minutes with apneic oxygenation.25,26
The success of apneic mass-movement oxygenation depends on airway patency to allow O2 to move into the apneic lungs. In the presence of air obstruction, not only does the lung gas volume decrease rapidly, but the intrathoracic pressure also decreases at a rate that is dependent on 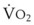 and thoracic compliance, subsequently leading to a marked fall in PaO2. When airway obstruction is relieved, rapid flow of O2 into the lungs occurs, and with high FIO2, rapid reoxygenation resumes.27
and thoracic compliance, subsequently leading to a marked fall in PaO2. When airway obstruction is relieved, rapid flow of O2 into the lungs occurs, and with high FIO2, rapid reoxygenation resumes.27
Apneic mass-movement oxygenation can be achieved by preoxygenation followed by insufflation of O2 through a nasopharyngeal or oropharyngeal cannula or through a needle inserted in the cricothyroid or cricotracheal membrane. This provides at least 10 minutes of adequate oxygenation in healthy apneic patients whose airways are unobstructed and therefore has many practical applications.31 In patients who are difficult to intubate or ventilate, pharyngeal O2 insufflation (or tracheal insufflation, in cases of upper airway obstruction) may allow additional time for laryngoscopy and endotracheal intubation.31–33 This can be advantageous in patients who have decreased O2 reserves, such as children, pregnant women, obese patients, and patients with adult respiratory distress syndrome (ARDS).30,32 The combination of preoxygenation and apneic diffusion oxygenation can be used during bronchoscopy and can provide the otolaryngologist with adequate time for glottic surgery unimpeded by the presence of the endotracheal tube (ETT) or by the patient’s respiratory movements.33
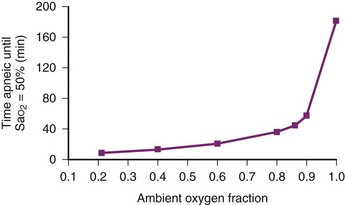
During apneic diffusion oxygenation via an open airway, the increase in time to Hb desaturation achieved by increasing the FIO2 from 0.9 to 1.0 is greater than that caused by increasing the FIO2 from 0.21 to 0.9. Increasing the FIO2 from 0.9 to 1.0 more than doubles the time to desaturation34 (Fig.13-2).
IV Efficacy and Efficiency of Preoxygenation
Studies of preoxygenation have focused on measurements of indices reflecting its efficacy and efficiency.18 Measurements of alveolar O2,14,35,36 alveolar N2,37 or PaO2 reflect the efficacy of preoxygenation, whereas the decline of SaO2 during apnea is indicative of its efficiency.9,16,18,37,38
The SaO2 may be misleading as a guide to alveolar denitrogenation. A saturation of 100% measured by pulse oximetry (SpO2) is most definitely not a reason to stop denitrogenation and may occur well before the lungs are adequately denitrogenated. Conversely, failure of SpO2 to increase substantially during denitrogenation does not necessarily indicate failure of preoxygenation or lack of its value; patients with substantial pulmonary shunting may achieve excellent pulmonary O2 reservoirs while remaining hypoxemic.39
A Efficacy of Preoxygenation
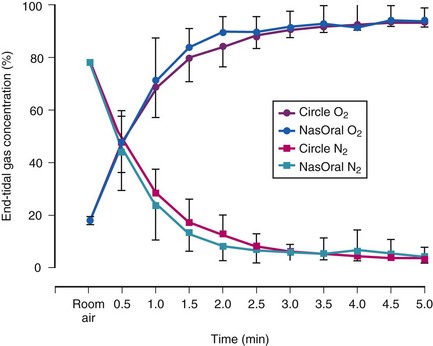
Preoxygenation increases the alveolar O2 and decreases the alveolar N2 in a parallel fashion (Fig. 13-3).37,40 It is the washout of N2 from the lungs that is the key to achieving preoxygenation.37,40 The terms preoxygenation and denitrogenation have been used synonymously to describe the same process, although a change in focus from preoxygenation to denitrogenation has been suggested.37
1. The anesthesia circuit is flushed by a high O2 flow.
2. A nonleaking face mask is used to avoid air entrainment.
3. An O2 flow of 5 L/min is required for TVB, and a flow of 10 L/min is necessary for the deep breathing.
The end points of maximal alveolar preoxygenation or denitrogenation have been defined as an end-tidal O2 concentration (EtO2) of approximately 90% and an end-tidal nitrogen concentration (EtN2) of 5%.15,35,36 In an adult with a normal FRC and VO2, an EtO2 greater than 90% means that the lungs contain more than 2000 mL of O2 (8 to 10 times the VO2).27 Because of the obligatory presence of CO2 and water vapor in the alveolar gas, an EtO2 greater than 97% cannot be easily achieved. Factors affecting the efficacy of preoxygenation include FIO2, duration of breathing, and the 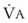 /FRC ratio (Box 13-1).
/FRC ratio (Box 13-1).
Box 13-1 Factors Affecting the Efficacy and Efficiency of Preoxygenation
1 Fraction of Inspired Oxygen
The main reasons for failure to achieve an FIO2 close to 1.0 are a leak under the face mask,14,18,41–43 rebreathing of exhaled gases, and the use of systems incapable of delivering a high O2 concentration, such as resuscitation bags.40 Even the presence of minor leaks may not be fully compensated for by increasing the fresh gas flow (FGF) or by increasing the duration of preoxygenation. Bearded patients, edentulous patients, patients with sunken cheeks, the presence of nasogastric tubes, use of a wrong face mask size, improper use of head straps, and use of systems allowing air entrainment under the face mask are all common factors causing leaks between the face mask and the patient’s head resulting in lower FIO2. Clinical end points indicative of a sealed system are movement of the reservoir bag in and out with inhalation and exhalation, presence of a normal capnogram and end-tidal CO2, (EtCO2), and measurements of inspired and EtCO2 values.18
There is reluctance among some anesthesiologists to use preoxygenation routinely because the mask presents discomfort to the patient and some patients find preoxygenation objectionable. There is clearly marked overestimation of the patient’s discomfort by the anesthesiologist. In fact, patients’ discomfort during preoxygenation is not more than the discomfort during other procedures such as the placement of intravenous lines.44,45 It is our experience that patients accept a tight-fitting mask if the procedure is explained beforehand and they are told that “it is important to fill the tank with oxygen.”
Although anesthetic circuits can deliver 100% O2 concentration, the FIO2 can be influenced by the type of breathing, the level of FGF, and the duration of breathing.17 In a study involving volunteers that compared preoxygenation techniques using a semiclosed circle absorber with varying FGF in the same subjects, it was found that with TVB, inspired O2 concentration was 95% with FGF of 5 L/min and increased to 98% with FGFs of 7 and 10 L/min. However, with deep breathing, the inspired O2 concentration was only 88% at 5 L/min, 91% at 7 L/min, and 95% at 10 L/min FGF (Fig. 13-4).17 These findings imply that increasing the FGF from 5 to 10 L/min has little impact on increasing FIO2 during TVB but has a noticeable effect during deep breathing.17 Because of the breathing characteristics of the circle system, the minute ventilation during deep breathing may exceed the FGF, resulting in rebreathing of exhaled gases (N2) and consequently decreasing the FIO2; in contrast, during TVB, rebreathing of exhaled gases is negligible, and increasing the FGF from 5 to 10 L/min would have only a slight effect on FIO2.12,17
2 Duration of Breathing, Functional Residual Capacity, and Alveolar Ventilation
Sufficient time is needed to accomplish maximal preoxygenation. With an FIO2 close to 1, most healthy adult patients can reach the target level of EtO2 greater than or equal to 90% (or EtN2 ≤ 5%) within 3 to 5 minutes of TVB. The half-time for exponential change in fraction of alveolar O2 concentration (FAO2) with a step change in FIO2 for a nonrebreathing system is given by the equation, FAO2 = 0.693 × VFRC/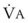 . (VFRC is volume of functional residual capacity). With a VFRC of 2.5 L, the half-times are 26 seconds when
. (VFRC is volume of functional residual capacity). With a VFRC of 2.5 L, the half-times are 26 seconds when 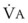 = 4 L/min and 13 seconds when
= 4 L/min and 13 seconds when 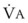 = 8 L/min.18 Therefore, most of the O2 that can be stored in the lungs may be brought in by hyperventilation with an FIO2 of 1.0 for a shorter period of time than that needed with TVB.18 This is the basis for the deep breathing techniques, which have been introduced as an alternative to TVB.9,18
= 8 L/min.18 Therefore, most of the O2 that can be stored in the lungs may be brought in by hyperventilation with an FIO2 of 1.0 for a shorter period of time than that needed with TVB.18 This is the basis for the deep breathing techniques, which have been introduced as an alternative to TVB.9,18
Changes in 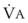 and FRC can have a marked effect on the rate of rise in EtO2 (and decrease in EtN2) during preoxygenation. Because of their increased
and FRC can have a marked effect on the rate of rise in EtO2 (and decrease in EtN2) during preoxygenation. Because of their increased 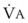 and decreased FRC, EtO2 rises faster in pregnant women than in nonpregnant women.14,46,47 Similarly, preoxygenation can be accomplished faster in infants and children than in adults.48,49
and decreased FRC, EtO2 rises faster in pregnant women than in nonpregnant women.14,46,47 Similarly, preoxygenation can be accomplished faster in infants and children than in adults.48,49
B Efficiency of Preoxygenation
Preoxygenation can markedly delay arterial oxyhemoglobin desaturation during apnea. In healthy individuals breathing air, desaturation to 70% can occur within 1 minute, whereas with adequate preoxygenation, desaturation occurs after 5 minutes. The delay in desaturation during apnea depends on the efficacy of preoxygenation, the capacity for O2 loading, and 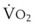 (see Box 13-1). Patients with a decreased capacity for O2 loading (decreased FRC, PaO2, CaO2, or cardiac output) or with increased
(see Box 13-1). Patients with a decreased capacity for O2 loading (decreased FRC, PaO2, CaO2, or cardiac output) or with increased 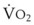 , or both, desaturate much faster during apnea than healthy patients do.18,29,42,50–52 The main difference in the rate of apnea-induced oxyhemoglobin desaturation after different preoxygenation techniques is observed between SaO2 levels of 100% and 99%.18,27,29,34,50 This range represents the flat portion of the oxyhemoglobin dissociation curve. When O2 reserves are depleted, rapid desaturation occurs regardless of the technique of preoxygenation and is similar to that observed in patients breathing air.
, or both, desaturate much faster during apnea than healthy patients do.18,29,42,50–52 The main difference in the rate of apnea-induced oxyhemoglobin desaturation after different preoxygenation techniques is observed between SaO2 levels of 100% and 99%.18,27,29,34,50 This range represents the flat portion of the oxyhemoglobin dissociation curve. When O2 reserves are depleted, rapid desaturation occurs regardless of the technique of preoxygenation and is similar to that observed in patients breathing air.
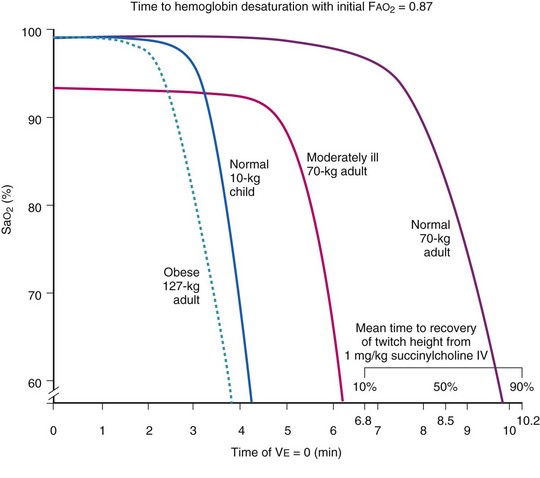
Farmery and Roe developed a computer model describing the rate of oxyhemoglobin desaturation during apnea.50 This model was found to agree reasonably well with actual data from patients whose weight and degree of normalcy and preoxygenation were reliably known (Fig. 13-5).29,50 Because it would be dangerous to obtain data in time to marked oxyhemoglobin desaturation in humans, this model is uniquely useful for analysis of oxyhemoglobin desaturation below 90%.29,50 As the preapnea FAO2 is progressively decreased from 0.87 to 0.8, 0.7, 0.6, 0.5, 0.4, 0.3, and 0.13 (air) in a healthy 70-kg patient, the apnea time to 60% SaO2 is progressively decreased from 9.9 to 9.31, 8.38, 7.30, 6.37, 5.40, 4.40, 3.55, and 2.8 minutes, respectively.29,50 Figure 13-5 shows that for a healthy 70-kg adult, a moderately ill 70-kg adult, a healthy 10-kg child, and an obese 127-kg adult, 80% SaO2 is reached after 8.7, 5.5, 3.7, and 3.1 minutes, respectively, whereas 60% SaO2 is reached at 9.9, 6.2, 4.23, and 3.8 minutes, respectively.18,29
V Techniques of Preoxygenation
Two main techniques are used: TVB and deep breathing (Box 13-2).
Box 13-2 Techniques of Preoxygenation
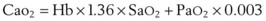
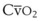 ) when there is a mixed venous O2 tension (
) when there is a mixed venous O2 tension (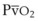 ) of 40 mm Hg and mixed venous oxyhemoglobin saturation (
) of 40 mm Hg and mixed venous oxyhemoglobin saturation (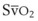 ) of 75% can be calculated accordingly.
) of 75% can be calculated accordingly.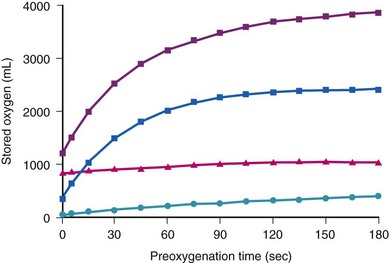
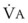 ) to FRC. Because the O2 flow used for
) to FRC. Because the O2 flow used for 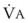 is delivered via an anesthesia circuit, preoxygenation occurs in two sequential stages according to the time constant, which is the time required for a flow through a container (volume) to equal the capacity. These two stages are
is delivered via an anesthesia circuit, preoxygenation occurs in two sequential stages according to the time constant, which is the time required for a flow through a container (volume) to equal the capacity. These two stages are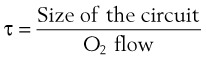
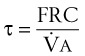
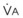 /FRC
/FRC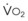
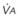 FRC, ratio of alveolar ventilation to functional residual capacity;
FRC, ratio of alveolar ventilation to functional residual capacity; 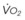 , O2 consumption.
, O2 consumption.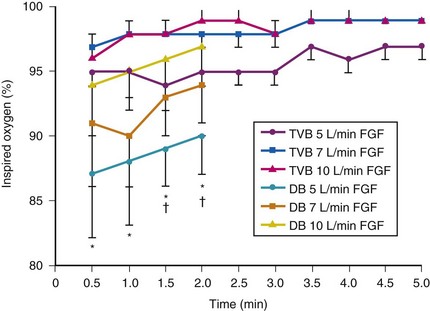
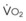 ]), and in a moderately ill adult, compared with a healthy adult. F
]), and in a moderately ill adult, compared with a healthy adult. F







