John Carlisle This chapter will describe: • cardiovascular disease • the role of testing in the preoperative phase • the clinical approach to preoperative optimisation of cardiovascular disease • therapeutic treatment of cardiovascular disease and implications for perioperative care. One in ten adults in the United Kingdom have chronic cardiovascular disease. The most common disease process is atherosclerosis – fatty scarring within arterial walls. This inflammatory process thickens the arterial wall (throttling blood supply – angina, claudication), which becomes stiff (systolic hypertension) and weak (aneurysm formation and wall dissection). However, arterial narrowing, wall stiffness and weakness do not directly cause blood clots that cause most atherosclerotic deaths. Fat, leaking through scabs (plaques) that cover diseased arterial walls, causes blood to clot. The clot stops blood flow, causing tissue infarction (heart attacks, strokes, black toes) and organ failure (heart failure, dementia and immobility). The risk of infarction is related to this risk of plaque rupture, which in turn depends upon the shape, position, fat and neutrophil content of the plaque. Atherosclerosis affects all arteries: symptomatic (ischaemia, infarction) and asymptomatic (plaques, intima-media thickness, stiffness) disease in one artery, for instance the carotid artery, predicts risk of symptomatic disease in the perfusion territory of other arteries, for instance the coronaries. About 1 in 30 adults have stable angina, 1 in 50 have had a heart attack and 1 in 70 have had a stroke. Heart attacks and strokes are becoming less common. The age-specific rate of cardiovascular events is 35% of the rate 25 years ago. Each year the rate of cardiovascular events in the population is 96% of the year before.1 The reduction in the rate of ischaemic death has not been accompanied by an increase in the rate of heart failure.2 The incidence of cardiovascular events increases with age. The risk parallels the overall risk of dying (see Chapter 2): the risk doubles every 7 years, or is 1.1 times the risk for someone a year younger. In the preoperative clinic, assessment of cardiovascular disease centres on a targeted history for heart attacks (myocardial infarction (MI)), angina, shortness of breath, stroke, transient cerebral ischaemia and claudication. Assessment is completed by an objective measure of fitness. If history and fitness measures are complete, the only physical examination required is heart auscultation for the murmur of aortic stenosis. Most preoperative assessment clinics would choose to refer patients back to general practitioners if they complain of cardiovascular symptoms that have not been previously addressed (see below). In Chapter 2 a Step process for calculating mortality risk is described in detail. This method can also be used to assess mortality risk in adult patients with: heart failure,3–11 valvular disease12 and acyanotic congenital heart disease.13–16 The absolute risk for having a stroke or developing heart disease depends upon age, sex, socioeconomic status and fitness. Smoking, hypertension, hypercholesterolaemia and diabetes may also be used to calculate risk, particularly if fitness has not been objectively measured. These are the same risk factors that predict all-cause mortality. There are various scores and risk calculators that you can use to calculate the risk of cardiovascular events.17,18 Fortunately the risk of non-fatal stroke, and the risk of non-fatal myocardial infarction, are each about half the calculated mortality risk.19 For instance, if the risk of dying per month is 1 in 500, the risk of having a non-fatal stroke will be about 1 in 1000, and the risk of having a non-fatal myocardial infarction will also be about 1 in 1000. So all one need do is calculate the risk of dying – as described in Chapter 2 – and divide by two to determine the risks of stroke and MI. Patients who have had strokes are more likely to have another stroke than an MI and vice versa. To calculate the risks of recurrent strokes, or recurrent MIs, multiply this crude estimate by 1.5. There are a number of common clinical conditions that are commonly managed in the preoperative clinic and described below. Hypertension was historically seen as a potentially dangerous risk factor, although in preoperative terms there is little evidence to support cancelling surgery due to the condition.20 Therefore, the decision whether to proceed with surgery or delay, in an attempt to reduce risk, should be made with the patient rather than on their behalf. Patients are made to endure painful and distressing symptoms despite their willingness to have surgery sooner, perhaps at slightly higher risk, rather than have surgery later in the hope that risk can be substantially reduced. Smoking and hypercholesterolaemia increase the risk of death, stroke and acute coronary syndrome by an amount similar to hypertension. But most clinicians do not postpone surgery until the cholesterol is reduced or until the patient has quit smoking for two months. We do not make patients walk, run, cycle or swim daily before surgery, yet fitness is a stronger predictor for death and morbidity than any other modifiable factor! Preventative medicine considers absolute risk to be much more important than relative risk. People with lower absolute risks are not treated (threshold is currently a 1 in 5 risk for a cardiovascular event over the next 10 years), even though ‘normal’ cardiovascular risk can also be reduced by lowering blood pressure or cholesterol concentration further. A normotensive man is more likely to die than a hypertensive woman the same age and is more likely to die than a hypertensive man 7 years younger. Normotensive normocholesterolaemic old men will have a greater absolute benefit from antihypertensive medication (and anticholesterolaemic medication) than young hypertensive hypercholesterolaemic women. Preventative medicine also recognises patient autonomy – you don’t have to take antihypertensive medication if you don’t want it. The common preoperative practice of postponing operations if the diastolic blood pressure exceeds 110mmHg is absurd without reference to absolute risk and patient autonomy. If a patient does not want their blood pressure reduced to limit non-operative long-term risk there is little reason to insist that they have their blood pressure reduced for an operation. One reason that blood pressure has become a perioperative issue is that it is the only cardiovascular risk factor, apart from fitness, that can be easily measured anywhere. But ironically blood pressure is the only variable that systematically overestimates risk when measured in hospital. The blood pressure measurements that correlate best with cardiovascular events are those taken outside hospital.21 The other reason that blood pressure has become a preoperative issue above all other cardiovascular risk factors is that it is measured intraoperatively and anaesthetic-induced hypotension is more likely in hypertensive patients. This may be a good reason to alter anaesthetic management for patients with a history of hypertension, but it is not a good reason to cancel hypertensive patients. Only one in four patients prescribed antihypertensive medication become normotensive.22 Small reductions in blood pressure can reduce stroke risk, but have little effect on heart attack risk and do not prevent deaths.23 The absolute benefit from treating hypertension will vary, depending upon patient age, sex, fitness and previous cardiovascular events. Between 5000 and 12,000 hypertensive adults who have not had a cardiovascular event need to be treated to prevent one stroke per month.24 Beta-blockers decrease blood pressure by 11/6mmHg on average. One stroke per month is prevented for every 12,000 hypertensive patients treated with a beta-blocker.25 Calcium channel blockers decrease blood pressure by similar amounts but prevent three strokes per month for every 12,000 hypertensive patients treated.26 One myocardial infarction per month is prevented for every 12,000 hypertensive patients who take aspirin. However, overall mortality is not decreased by aspirin because the risk for fatal haemorrhage is increased by aspirin.27 Diuretics probably reduce blood pressure the quickest.28 The possible reduction in stroke risk provided by preoperative antihypertensive treatment for two months is similar to the increase in stroke risk associated with being two months older. It is time to remove the stigma of elevated blood pressure measured in preoperative assessment clinics. Global cardiovascular and mortality risks for each patient should be calculated without undue emphasis on hypertension. Myocardial infarction kills, mainly through ventricular fibrillation or heart failure. The risk of dying in the first month after a myocardial infarction is about 50 times the risk of dying in the month before a myocardial infarction, despite treatment (fibrinolysis or angioplasty).29 The long-term relative mortality risk associated with a previous myocardial infarction is 1.5 times the risk without. The claim – made in many perioperative texts – that risk has returned to ‘normal’ six months after a heart attack is certainly incorrect. By 12 months, the relative risk has fallen to about three times the risk of someone who did not have an MI. Between one and 12 months after a heart attack, the relative risk probably falls exponentially, so that six months after a heart attack the relative risk may be about 6 (see Chapter 2). The absolute risk of dying – which is the risk that will help the patient and clinicians decide when and whether to proceed with surgery – is calculated by combining the relative risk with the other risk factors described previously in Chapter 2. Age, history of cardiovascular events and decreased fitness explain most of the doubled mortality risk associated with atrial fibrillation (AF). Warfarin decreases the risk of dying in patients with atrial fibrillation (relative risk 0.8): if the monthly mortality risk for a hypothetical patient with AF is 5 in 1200, warfarin would reduce the risk to 4 in 1200. Warfarin halves the risk of stroke, for instance from 5 in 1200 per month to 2.5 in 1200 per month. Aspirin is less effective than warfarin, the relative risk for stroke being about 0.8 compared to placebo. Of course the absolute benefit from either warfarin or aspirin depends upon the absolute risk of death or stroke without medication.30–32 See above and Chapter 2 to calculate this baseline risk. The risk of symptomatic arterial thromboemboli is increased for patients with prosthetic heart valves, particularly within the first three months after valve replacement. But the absolute risk is less for biological valves (as opposed to mechanical valves) and aortic valve replacement (as opposed to mitral valve replacement). Patients with mechanical mitral valves have about a 1 in 1000 monthly risk of thromboemboli whilst taking warfarin. Patients who have biological aortic valves have about a 1 in 2400 monthly risk without any medication, but sometimes take aspirin.33 Before surgery the questions that have to be answered for patients taking warfarin, aspirin or clopidogrel (see below) for atrial fibrillation, prosthetic heart valves or intracoronary stents are: • What is the risk of death or disability from enclosed or massive haemorrhage for this surgery, both on medication and off medication? • What is the risk of death or disability from thromboemboli, both on medication and off medication? The answers to these questions should inform the decisions: • whether to stop anticoagulation before surgery; • whether to start alternative antithrombotic interventions perioperatively; • when to restart anticoagulation after surgery if it has been stopped. One typical preoperative protocol for warfarin is: Take the last dose of warfarin five days before all surgeries except dental and cataract extractions. (1) Start subcutaneous fractionated heparin 36 hours later at home (administered by patient, district nurse or practice nurse) if the clot risk is high; do not start heparin if the clot risk is low. See Table 5.1. (2) No fractionated heparin in the 12 hours preceding surgery. (3) Restart warfarin after surgery (the same day or as prescribed by the surgical consultant). Table 5.1 Factors associated with risk of clot
Chapter 5 Preoperative assessment of cardiovascular system and management of concurrent disease
SUMMARY
INTRODUCTION
Cardiovascular disease and cardiovascular outcomes
Hypertension
Myocardial infarction
Atrial fibrillation
Prosthetic heart valves
Anticoagulation
| HIGH risk of clot | LOW risk of clot |
| Within 30 days of first warfarin dose | At least 60 days after first warfarin dose |
| Treated for multiple arterial or venous clots | Treated for first clot |
| Atrial fibrillation with mitral valve disease | Atrial fibrillation without mitral valve disease |
| History of clot with cancer | |
| Mechanical mitral valve | Tissue valves and mechanical aortic valve |
Further discussion on preoperative haematological management including warfarin can be found in Chapter 11.
Coronary artery bypass and coronary artery stents
For most patients with chronic stable angina, whether mild, moderate or severe, coronary artery bypass grafting (CABG) and percutaneous coronary interventions (PCI) are palliative procedures. That is, they relieve anginal pain that has not responded to maximal medical therapy but they do not prolong survival.
Most of the evidence comparing medication with CABG comes from 2649 patients recruited into seven randomised controlled trials (RTCs) between 1972 and 1984.34 Drugs used to reduce cardiovascular risk and techniques used to achieve coronary bypass have changed since 1984 but further RCTs have not been conducted. The median survival was 100 months after CABG and 96 months without (difference unlikely to be less than 50 days), but more people died in the first year with CABG than without. It is not surprising that patients do not benefit from ‘prophylactic’ CABG before undergoing non-cardiac surgery.35,36
Percutaneous coronary angioplasty and stenting, collectively known as ‘percutaneous coronary intervention’ (PCI), have also been used to treat angina since the CABG studies were published. PCI is less invasive than CABG, but relief from angina is less prolonged.37–39 PCI, with or without stents, does not prolong survival in patients with stable angina and simple single-vessel disease.40,41 PCI probably shortens survival in patients with complex multivessel coronary disease: the risk of dying might be increased ten-fold in the first month after stenting and the risk of death remains two to three times expected for more than a year.42–44 Patients are about 1.2 times more likely to die after PCI (with or without stents) than after CABG.
Angioplasty inflates a balloon to stretch open a narrowed length of coronary artery. The opened artery can narrow, either due to elastic recoil (‘rebound’) or continued atherosclerotic hypertrophy. Bare metal mesh stents reduce recoil in the short term but wall hypertrophy through the mesh can narrow the vessel lumen. Mesh impregnated with a drug – sirolimus or paclitaxel – slows wall hypertrophy, maintaining coronary arterial blood flow. The risk of dying in the first three months following insertion of drug-eluting stents (DES) is possibly two-thirds the risk of dying after insertion of bare-metal stents (BMS). However, the risk of dying after this initial period is probably three times greater for DES than for BMS.45–51 This may be related to the effect of stopping clopidogrel, which increases the DES risk of dying four to five times but has little effect on the BMS risk of dying.52–57 The increased risk of dying in patients with DES who stop clopidogrel can be used to try and calculate the relative merits of continuing or stopping clopidogrel perioperatively.
For example, consider a 67-year-old man who had a single drug-eluting stent inserted 6 months ago in the left anterior descending coronary artery. He has not had a heart attack, a stroke, renal failure or peripheral vascular disease. He achieves a peak oxygen consumption of 21ml/kg per minute (expected 27ml/kg per minute). The average monthly risk of dying for a 67-year-old man is 1 in 600. The reduced exercise capacity increases this risk to about 1 in 300. Because the DES was inserted for simple disease and because he is still taking clopidogrel it is reasonable to assume that his risk of dying is about 1 in 300 per month. If the clopidogrel is stopped his risk of dying increases, perhaps five-fold, to about 1 in 60. The risk of dying in the month following laparoscopic cholecystectomy may be about twice the risk of dying in the month preceding surgery. So, his risk of dying in the month after surgery is about 1 in 150 if clopidogrel is continued and about 1 in 30 if it is stopped, with similar risks for non-fatal myocardial infarction.
Do not refer patients to cardiologists if they think their angina is adequately controlled. Refer patients who think their angina is inadequately controlled if:
• they are not on maximal medical therapy (refer to previous cardiology investigations and documentation);
• they are on maximal medical therapy but they will consider either PCI or CABG.
Do not refer patients who are on maximal medical therapy but will not consider PCI or CABG, or patients whose angiography has already shown anatomy that is not amenable to either technique.
Cardiovascular testing
Coronary angiography
Table 5.2 Matrix for relations between ISWT, CPX and cardiac symptoms
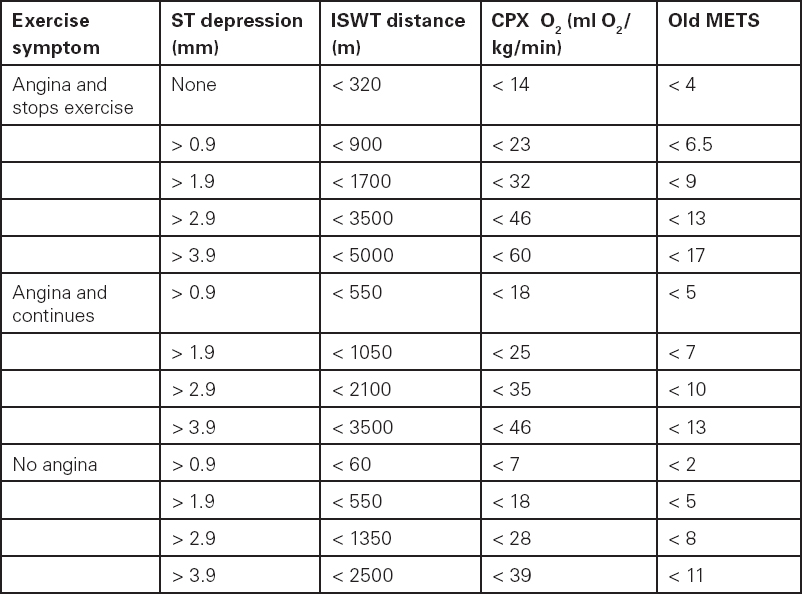
MET: metabolic equivalent (one MET = 3.5ml O2/kg/min). Modified from Mark et al.59
Current guidelines recommend angiography for patients with suspected coronary artery disease and a predicted monthly mortality greater than 1 in 400.58 However, the authors of this guideline had to have considered the fact that most men older than 71 years have at least this mortality risk. Most patients will not experience angina during preoperative exercise testing, including those with ischaemic heart disease and a history of angina – breathlessness is a better predictor for the presence of coronary artery stenoses than angina. The aim is to identify patients more likely to die than the average with ischaemic heart disease. The seven trials I mentioned above found that CABG increased median survival by 9 months, from 7 years 6 months to 8 years 3 months (difference unlikely to be less than 3 months) in the third of patients with higher risk, and by 19 months, from 6 years 9 months to 8 years 4 months (difference unlikely to be less than 5 months) in patients with left main stem coronary artery stenosis of at least 70%.
If a patient fails to achieve the following distances (see Table 5.2, page 95) during the incremental shuttle walk test (ISWT) or the following oxygen consumptions during cardiopulmonary exercise testing (CPX), and has ST changes compared to rest, the guideline suggests referral for coronary angiography.
Again there is little to be gained by cardiological review if the patient has had coronary angiography that has shown that PCI and CABG would not be helpful.
Echocardiograms
Fitness, measured by either ISWT or CPX, predicts survival more accurately than does transthoracic echocardiography. Even dobutamine stress echocardiography, single photon emission computerised tomography or stress scintillography probably add little prognostic information to CPX results.
Patients with left ventricular outflow obstruction, such as severe aortic stenosis, can be anaesthetised for non-cardiac surgery. One might expect that risk could be estimated by fitness tests, because patients whose cardiac output is limited will reach lower power levels. It is unknown whether fitness tests adequately assess both non-operative and operative risks in patients with undiagnosed aortic stenosis. Because the answer is uncertain, and because tailored intraoperative management may reduce risk, it seems reasonable to use echocardiography, in addition to exercise testing, to identify patients with moderate or severe aortic stenosis. I cannot guide readers on how useful different echocardiographic strategies are to measure and limit mortality risk. What follows is a strategy that has not been validated; your local policy will depend upon discussions with cardiologists and echocardiographers.
You could request an echocardiogram for a patient with a heart murmur who meets one or more of the following criteria:
• Left ventricular hypertrophy on a resting 12-lead electrocardiogram
• Inability to achieve 4 old METS or 5.5 new METs (peak oxygen consumption of 14mls O2/kg per min)
• Faints or angina
• Planned major surgery (if older than 40 years)
• Planned hypotensive anaesthesia (if older than 40 years).
Unprepared elective or scheduled patients
This chapter has concentrated on cardiovascular risk assessment and disease management in patients presenting for elective or scheduled surgery who are under the care of a primary care physician. The principles that have been outlined also apply to patients who are either not registered with, or who have not attended, a primary care service. To assess non-operative risks you will need to spend more time either directly assessing risk or liaising with a primary care service. Unprepared patients are more likely to benefit from delaying surgery to allow risk factors to be assessed and reduced, but perhaps are also less likely to want to do so.
The principles of assessment and preparation of patients for emergency surgery are also the same. But risk reduction will depend upon reducing the relative risk of surgery as there will be no time to reduce non-operative risk.
Management of disease, management of risk
This chapter has discussed aspects of managing cardiovascular disease and reducing risk. It seems reasonable to use the opportunity of the visit to the preoperative assessment clinic to educate the patient about interventions that reduce the risk of death, stroke and heart attack in primary care. It is also right to identify patients who fulfil criteria for primary (or secondary) drug prevention of cardiovascular events but who are not receiving full pharmaceutical protection. At the time of writing there is inadequate evidence to support starting medication (such as beta-blockers or statins) just because the patient is scheduled for surgery.
CONCLUSION
Assessment of cardiovascular disease depends upon a targeted history, a fitness test and cardiac auscultation. Perioperative risk of death and cardiovascular events is calculated from the preoperative risk of death, taking into account the results of assessment. Particular attention should be paid to coronary stent implantation.
For many patients cardiovascular disease is optimally managed, and risk reduced, by primary care. The preoperative clinic can assist primary care by checking that patients are aware of how they can reduce their long-term risk, by stopping smoking, exercise, a balanced diet and drugs (if the 10-year risk for a cardiovascular event exceeds 1 in 5). Of particular note, blood pressure measurements in secondary care are unlikely to realistically reflect long-term cardiovascular risk. Delaying surgery to commence treatment in patients who are truly hypertensive is unlikely to result in significant net perioperative benefit. Delaying surgery to get fitter will be more beneficial.
Stopping clopidogrel increases the risk for postoperative in-stent thrombosis, myocardial infarction and death, by about 1.2 times for bare-metal stents and 5 times for drug-eluting stents.
REFERENCES
1. S.L. Hardoon, P.H. Whincup, L.T. Lennon, S.G. Wannamethee, S. Capewell and R.W. Morris (2008). How much of the recent decline in the incidence of myocardial infarction in British men can be explained by changes in cardiovascular risk factors? Circulation 117: 598–604.
2. M.J. Goldacre, D. Mant, M. Duncan and M. Griffith (2005). Mortality from heart failure in an English population, 1979-2003: study of death certification. Journal of Epidemiology and Community Health 59: 782–4.
3. M. Guazzi, G. Reina, G. Tumminello and M.D. Guazzi (2005). Exercise ventilation inefficiency and cardiovascular mortality in heart failure: the critical independent prognostic value of the arterial CO2 partial pressure. European Heart Journal 26: 472–80.
4. M. Robbins, G. Francis, F.J. Pashkow, C.E. Snader, K. Hoercher, J.B. Young et al. (1999). Ventilatory and heart rate responses to exercise better predictors of heart failure mortality than peak oxygen consumption. Circulation 100: 2411–7.
5. L.C. Davies, R. Wensel, P. Georgiadou, M. Cicoira, A.J.S. Coats, M.F. Piepoli et al. (2006). Enhanced prognostic value from cardiopulmonary exercise testing in chronic heart failure by non-linear analysis: oxygen uptake efficiency slope. European Heart Journal 27: 684–90.
6. U. Corrà, A. Mezzani, E. Bosimini and P. Giannuzzi (2004). Cardiopulmonary exercise testing and prognosis in chronic heart failure: a prognosticating algorithm for the individual patient. Chest 126: 942–50.
7. P. Agostoni, M. Guazzi, M. Bussotti, S. De Vita and P. Palermo (2002). Carvedilol reduces the inappropriate increase in ventilation during exercise in heart failure patients. Chest 122: 2062–7.
8. A.K. Gitt, K. Wasserman, C. Kilkowski, T. Kleeman, A. Kilkowski, M. Bangert et al. (2002). Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation 106: 3079–84.
9. M. Guazzi, J. Myers and R. Arena (2005). Cardiopulmonary exercise testing in the clinical and prognostic assessment of diastolic heart failure. Journal of the American College of Cardiology 46: 1883–90.
10. T. Bilsel, S. Terzi, T. Akbulut, N. Sayar, G. Hobikoglu and K. Yesilcimen(2006). Abnormal heart rate recovery immediately after cardiopulmonary exercise testing in heart failure patients. International Heart Journal 47: 431–40.
11. M. Arzt, M. Schulz, R. Wensel, S. Montalvàn, F.C. Blumberg, G.A.J. Riegger et al. (2005). Nocturnal continuous positive airway pressure improves ventilatory efficiency during exercise in patients with chronic heart failure. Chest 127: 794–802.
12. D. Messika-Zeitoun, B.D. Johnson, V. Nkomo, J-F. Avierinos, T.G. Allison, C. Scott et al. (2006). Cardiopulmonary exercise testing determination of functional capacity in mitral regurgitation. Journal of the American College of Cardiology 47: 2521–7.
13. K. Dimopoulos, D.O. Okonko, G-P. Diller, C.S. Borberg, T.V. Salukhe, S.V. Babu-Narayan et al. (2006). Abnormal ventilatory response to exercise in adults with congenital heart disease relates to cyanosis and predicts survival. Circulation 113: 2796–802.
14. A. Giardini, S. Specchia, T.A. Tacy, G. Coutsoumbas, G. Gargiulo, A. Donti et al. (2007). Usefulness of cardiopulmonary exercise to predict long-term prognosis in adults with repaired tetralogy of Fallot. American Journal of Cardiology 99: 1462–7.
15. H. Tsurugaya, A. Adachi, M. Kurabayashi, S. Ohshima and K. Taniguchi (2006). Prognostic impact of ventilatory efficiency in heart disease patients with preserved exercise tolerance. Circulation Journal 70: 1332–6.
16. A. Koike, H. Itoh, M. Kato, H. Sawada, T. Aizawa, L. Tai Fu et al. (2002). Prognostic power of ventilatory responses during submaximal exercise in patients with chronic heart disease. Chest 121: 1581–8.
17. J. Hippisley-Cox, C. Coupland, Y. Vinogradova, J. Robson, M. May and P. Brindle (2007). Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. British Medical Journal 335: 136–48.
18. R.B. D’Agostino, R.S. Vasan, M.J. Pencina, P.A. Wolf, M. Cobain, J.M. Massaro et al. (2008). General cardiovascular risk profile for use in primary care. Circulation 117: 743–53.
19. P.G. Steg, D.L. Bhatt, P.W.F. Wilson, R. D’Agostino, E.M. Ohman, J. Röther et al. (2007). One-year cardiovascular event rates in outpatients with atherothrombosis. Journal of the Americal Medical Association 297: 1197–206.
20. S.J. Howell, J.W. Sear and P. Foëx (2004). Hypertension, hypertensive heart disease and perioperative cardiac risk. British Journal of Anaesthesia 92: 570–83.
21. R.H. Fagard and V.A. Cornelissen (2007). Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. Journal of Hypertension 25: 2193–8.
22. M. Burnier (2002). Blood pressure control and the implementation of guidelines in clinical practice: can we fill the gap? Journal of Hypertension 20: 1251–3.
23. J.A. Staessen, J.G. Wang and L. Thijs (2003). Cardiovascular prevention and blood pressure reduction: a quantitative overview updated until 1 March 2003. Journal of Hypertension 21: 1055–76
24. A. Quan, K. Kerlikowske, F. Gueyffier, J.P. Boissel, for the INDANA Investigators (2000). Pharmacotherapy for hypertension in women of different races. Cochrane Database of Systematic Reviews, Issue 2. Art. No.: CD002146. DOI: 10.1002/14651858. CD002146.
25. C.S. Wiysonge, H. Bradley, B.M. Mayosi, R. Maroney, A. Mbewu, L.H. Opie et al. (2007), Beta-blockers for hypertension. Cochrane Database of Systematic Reviews, Issue 1. Art. No.: CD002003. DOI: 10.1002/14651858.CD002003.pub2.
26. B. Dahlöf, P.S. Sever, N.R. Poulter, H. Wedel, D.G. Beevers, M. Caulfield et al. (2005). ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 366: 895–906.
27. G.Y.H. Lip and D.C. Felmeden (2004). Antiplatelet agents and anticoagulants for hypertension. Cochrane Database of Systematic Reviews, Issue 3. Art. No.: CD003186. DOI: 10.1002/14651858.CD003186.pub2.
28. J.P. Baguet, B. Legallicier, P. Auquier and S. Robitail (2007). Updated meta-analytical approach to the efficacy of antihypertensive drugs in reducing blood pressure. Clinical Drug Investigation 27: 735–53.
29. E. Boersma and the PCAT-2 trialist collaborative group (2006). Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. European Heart Journal 27: 779–88.
30. M.I. Aguilar and R. Hart (2005) Oral anticoagulants for preventing stroke in patients with non-valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database of Systematic Reviews, Issue 3. Art. No.: CD001927. DOI: 10.1002/14651858.CD001927.pub2.
31. V. Fuster, L.E. Ryden, D.S. Cannom, H.J. Crijns, A.B. Curtis, K.A. Ellenbogen et al. (2006). ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Circulation 114: e257-e354.
32. S.H. Little and D.R. Massel (2003). Antiplatelet and anticoagulation for patients with prosthetic heart valves. Cochrane Database of Systematic Reviews, Issue 4. Art. No.: CD003464. DOI: 10.1002/14651858.CD003464.
33. R.O. Bonow, B.A. Carabello, K. Chatterjee, A.C. De Leon, D.P. Faxon, M.D. Freed et al. (2006). ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. Journal of the American College of Cardiology 48: e1-e148.
34. S. Yusuf, D. Zucker, P. Peduzzi, L.D. Fisher, T. Takaro, J.W. Kennedy et al. (1994). Effect of coronary artery bypass graft surgery on survival: Overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet 344: 563–70.
35. E.O. McFalls, H.B. Ward, T.E. Moritz, S. Goldman, W.C. Krupski, F. Littooy et al. (2004). Coronary-artery revascularization before major vascular surgery. New England Journal of Medicine 351: 2795–804.
36. D. Poldermans, O. Schouten, R. Vidakovic, J.J. Bax, I.R. Thomson, S.E. Hoeks et al. (2007). A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: The DECREASE-V Pilot Study. Journal of the American College of Cardiology 49: 1763–9.
37. D.M. Bravata, A.L. Glenger, K.M. McDonald, V. Sundaram, M.V. Perez, R. Varghese et al. (2007). Systematic review: the comparative effectiveness of percutaneous coronary interventions and coronary artery bypass graft surgery. Annals of Internal Medicine 147: 703–16.
38. E.L. Hannan, M.J. Racz, G. Walford, R.H. Jones, T.J. Ryan, E. Bennett et al. (2005). Long-term outcomes of coronary artery bypass grafting versus stent implantation. New England Journal of Medicine 352: 2174–83.
39. E.L. Hannan, C. Wu, G. Walford, A.T. Culliford, J.P. Gold, C.R. Smith et al. (2008). Drug-eluting stents vs. coronary-artery bypass grafting in multivessel coronary disease. New England Journal of Medicine 358: 331–41.
40. W.E. Boden, R.A. O’Rourde, K.K. Teo, P.M. Hartigan, D.J. Maron, W.J. Kostuk et al. (2007). Optimal medical therapy with or without PCI for stable coronary disease. New England Journal of Medicine 356: 1503–16.
41. D.G. Katritsis and J.P.A. Ioannidis (2005). Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: a meta-analysis. Circulation 111: 2906–12.
42. H.K. Win, A.E. Caldera, K. Maresh, J. Lopez, C.S. Rihal, M.A. Parikh et al. (2007). Clinical outcomes and stent thrombosis following off-label use of drug-eluting stents. Journal of the American College of Cardiology 297: 2001–9.
43. O.C. Marroquin, F. Selzer, S.R. Mulukutla, D.O. Williams, H.A. Vlachos, R.L. Wilensky et al. (2008). A comparison of bare-metal and drug-eluting stents for off-label indications. New England Journal of Medicine 358: 342–52.
44. G.W. Stone, S.G. Ellis, L. Cannon, J.T. Mann, J.D. Greenberg and D. Spriggs (2005). Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease. Journal of the Americal Medical Association 294: 1215–23.
45. G. Weisz, M.B. Leon, D.R. Holmes, D.J. Kereiakes, M.R. Clark, B.M. Cohen et al. (2006). Two-year outcomes after sirolimus-eluting stent implantation. Journal of the American College of Cardiology 47: 1350–5.
46. M-C. Morice, P.W. Serruys, J.E. Sousa, J. Fajadet, E.B. Hayashi, M. Perin et al. (2002). A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. New England Journal of Medicine 346: 1773–80.
47. J.W. Moses, M.B. Leon, J.J. Popma, P.J. Fitzgerald, D.R. Holmes, C. O’Shaughnessy et al. (2003). Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. New England Journal of Medicine 349: 1315–23.
48. G.W. Stone, S.G. Ellis, D.A. Cox, J. Hermiller, C. O’Shaughnessy, J.T. Mann et al. (2004). A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. New England Journal of Medicine 350: 221–31.
49. C. Spaulding, J. Daemen, E. Boersma, D.E. Cutlip and P.W. Serruys (2007). A pooled analysis of data comparing sirolimus-eluting stents with bare-metal stents. New England Journal of Medicine 356: 989-97.
50. A. Kastrati, A. Dibra, C. Spaulding, G.J. Laarman, M. Menichelli, M. Valgimigli et al. (2007). Meta-analysis of randomized trials on drug-eluting stents vs. bare-metal stents in patients with acute myocardial infarction. European Heart Journal 28: 2706–13.
51. B. Lagerqvist, S.K. James, U. Stenestrand, J. Lindbäck, T. Nilsson and L. Wallentin (2007). Long-term outcomes with drug-eluting versus bare-metal stents in Sweden. New England Journal of Medicine 356: 1009–19.
52. M. Pfisterer, H.P. Brunner-La Rocca, P.T. Buser, P. Rickenbacher, P. Hunziker, C. Mueller et al. (2006). Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents. Journal of the American College of Cardiology 48: 2584–91.
53. E.L. Eisenstein, K.J. Anstrom, D.F. Kong, L.K. Shaw, R.H. Tuttle, D.B. Mark et al. (2007). Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. Journal of the Americal Medical Association 297: 159–68.
54. J.A. Spertus, R. Kettelkamp, C. Vance, C. Decker, P.G. Jones, J.S. Rumsfield et al. (2006). Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation 113: 2803–9.
55. I. Iakovou, T. Schmidt, E. Bonizzoni, L. Ge, G.M. Sangiorgi, G. Stankovic et al. (2005). Incidence, predictors and outcome of thrombosis after successful implantation of drug-eluting stents. Journal of the Americal Medical Association 293: 2126–30.
56. P.K. Kuchulakanti, W.W. Chu, R. Torguson, P. Ohlmann, S-W. Rha, L.C. Clavijo et al. (2006). Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents. Circulation 113: 1108–13.
57. J. Machecourt, N. Danchin, J.M. Lablanche, J.M. Fauvel, J.L. Bonnet, S. Marliere et al. (2007). Risk factors for stent thrombosis after implantation of sirolimus-eluting stents in diabetic and nondiabetic patients. Journal of the American College of Cardiology 50: 501–8.
58. P.J. Scanlon, D.P. Faxon, A-M Audet, B. Carabello, G.J. Dehmer, K.A. Eagle et al. (1999). ACC/AHA Guidelines for Coronary Angiography: Executive summary and recommendations. Circulation 99: 2345–57.
59. D.B. Mark, L. Shaw, F.E. Harrell Jr, M.A. Hlatky, K.L. Lee and J.R. Bengtson (1991). Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. New England Journal of Medicine 325: 849–53.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






