CHAPTER 99 PREOPERATIVE AND POSTOPERATIVE NUTRITIONAL SUPPORT: STRATEGIES FOR ENTERAL AND PARENTERAL THERAPIES
Nutritional support is an integral part of trauma and critical care management. Its role has undergone a dramatic evolution over the past two decades as we have developed a deeper understanding of the complex inflammatory and metabolic pathways that accompany surgical stress. The manipulation of this stress response and its inherent catabolic reaction is the focus of emerging nutritional therapies.
PREOPERATIVE NUTRITION
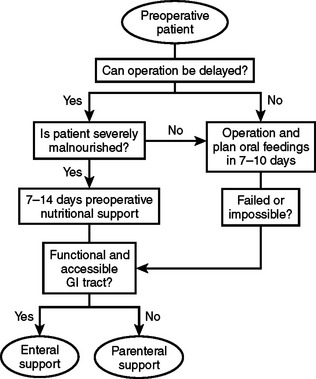
There are two circumstances in which preoperative nutritional support should be considered. One is for patients who will require major operative intervention, but cannot undergo immediate surgery and will have a prolonged fast for more than 5 days. The other circumstance is when operative intervention is delayed to treat patients with significant nutritional deficits that could increase postoperative morbidity (Figure 1).
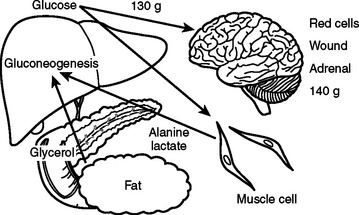
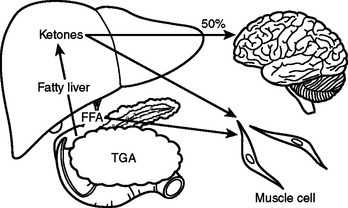
In the preoperative patient, the response to starvation is associated with a redistribution of substrate flow from peripheral tissues to meet metabolic demands. The falling level of insulin promotes the release of fatty acids and amino acids from adipose tissue and skeletal muscle. Although most peripheral tissues can utilize fatty acids as fuel, proteolysis continues to fuel gluconeogenesis in order to support the fuel requirements of the glucose dependent tissues (Figure 2). Over time, there is adaptation to starvation as the brain becomes able to use ketones for 50% of its fuel needs. As fat-derived fuel sources are utilized more, the dependence on protein catabolism decreases from 85% to 35% (Figure 3).
Patients with upper gastrointestinal tract malignancies have the highest incidence of protein-calorie malnutrition, with over 30% of patients demonstrating significant nutritional deficits. Preoperative chemotherapy and radiation, combined with cancer cachexia, obstruction, increased nutrient losses, and abnormal substrate metabolism, increase nutritional risk.1
Prospective studies have shown a decrease in major complications such as anastomotic leak and wound disruption when surgery is delayed and preoperative parenteral nutrition is administered to severely malnourished patients. However, there is an increase in infectious complications without clinical benefit when preoperative parenteral nutrition is administered to patients who are well nourished or onlymildly malnourished. It is important, therefore, to precisely define malnutrition to appropriately select patients for this treatment modality.2
Severe malnutrition can be diagnosed using a clinical nutritional evaluation tool, such as the Subjective Global Assessment.3 In 1982, Baker demonstrated the validity of a clinical assessment relative to one made on the basis of more objective laboratory values. The clinician uses historical information about recent food intake or unintentional weight loss and examines the patient for signs of nutritional depletion. Patients with multiple or severe stigmata of malnutrition or more than 15% weight loss within six months would be considered as seriously depleted. However, in patients with biopsy proven carcinoma, a weight loss of more than 10% in six months would indicate a high-risk group that would benefit from a course of preoperative nutritional support.
After selection of a patient for preoperative nutrition, it is necessary to decide on a formulation and treatment course. Although the optimal duration of therapy has yet to be determined, preoperative therapy from 7–15 days is standard. Total nonprotein calories should be calculated at 150% of basal energy expenditure as measured using indirect calorimetry or derived from the Harris-Benedict equations (Table 1). It is prudent to start patients with severe malnutrition and starvation at a basal energy rate for several days to prevent refeeding syndrome before increasing support to goal rates. After 3 days, support may be increased to 125% of basal requirements and then increased to goal as tolerated.
Table 1 Harris-Benedict Equations
Data from Van Way CW 3rd: Variability of the Harris-Benedict equation in recently published textbooks. J Parenter Enteral Nutr 16:566–568, 1992.
Preoperative Total Parenteral Nutrition
Total parenteral nutrition (TPN) should be administered to patients who are severely malnourished with nonfunctioning gastrointestinal tracts. Dextrose and lipid formulas are used to provide nonprotein calories, usually in a 70:30 ratio. The caloric values of TPN substrates can be found in Table 2. The amount of dextrose administered should be 4–6 mg/kg/min. However, in patients with chronic obstructive pulmonary disease or diabetes, it is recommended to keep the dextrose administration at 4 mg/kg/min or less. Blood sugars must be monitored and kept tightly controlled between 85 and 120 mg/dl.
Table 2 Caloric Value of Parenteral and Enteral Nutrients
| Nutrient | Parenteral (kcal/g) | Enteral (kcal/g) |
|---|---|---|
| Carbohydrate | 3.4 | 4.0 |
| Fat | 9.0 | 9.0 |
| Protein | 3.4 | 4.0 |
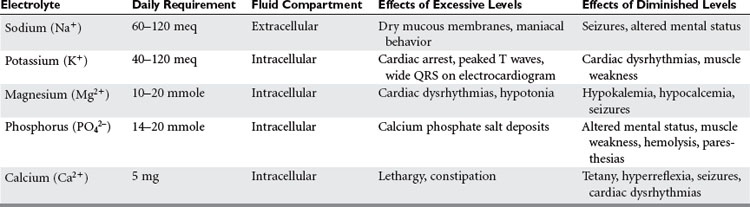
With protein-calorie malnutrition there is loss of the intracellular ions potassium, magnesium, and phosphorus, and a gain in sodium and water. During refeeding, sodium balance may become markedly positive and cause water retention. Potassium, phosphorus, and magnesium levels may drop precipitously upon initiation of nutritional support. It is important to monitor electrolytes and fluid balance to avoid the risk of refeeding syndrome. In addition, potassium and magnesium deficiencies must be corrected if anabolism is to occur (Table 3).
Trace minerals are inorganic compounds, and vitamins are complex organic compounds that regulate metabolic processes (Table 4). The majority act as coenzymes or as essential elemental constituents of enzyme complexes regulating the use of carbohydrates, proteins, and fats. Iron, zinc, copper, chromium, selenium, iodine, and cobalt are known to be necessary for health in man. However, in malnourished and seriously ill patients, requirements for zinc and selenium should be assessed and replenished as necessary.4
| Vitamin or Mineral | Function | Daily Requirement |
|---|---|---|
| Biotin | Coenzyme of carboxylase | 60 mcg |
| Chromium | Insulin utilization | 10–20 mcg |
| Copper | Enzyme systems and ceruloplasmin | 0.1–0.5 mcg |
| Folic acid | Nucleic acid synthesis | 600 mcg |
| Iron | Porphyrin-based compounds, enzymes, mitochondria | 0–2 mg |
| Niacin | Component of nicotinamide adenine dinucleotide and its phosphate (NADP) | 50 mg |
| Pantothenate | Component coenzyme A | 15 mg |
| Pyridoxine | Coenzyme of amino acid metabolism | 5 mg |
| Riboflavin | Coenzymes in redox enzyme system | 5 mg |
| Selenium | Component of glutathione peroxidase | 20–200 mcg |
| Thiamine (B1) | Cocarboxylase enzyme system | 5 mg |
| Vitamin A | Epithelial surfaces, retinal pigments | 2500 IU |
| Vitamin B12 | Nucleic acid synthesis | 12 mcg |
| Vitamin C | Redox reactions, collagen, immune function | 1000 mg |
| Vitamin D | Bone metabolism | – |
| Vitamin E | Membrane phospholipids | 50 IU |
| Vitamin K | Coagulation factors | 1–2 mg |
| Zinc | Enzyme systems | 1–15 mcg |
Preoperative Enteral Nutrition
The initial gastrointestinal barrier function is provided by mucous containing lactoferrin and lysozyme, both of which are effective, nonspecific inhibitors of microbial growth. Normal, undisturbed bacterial flora exert a similar effect. Epithelial tight junctions form the next line of nonspecific defense, with junctional integrity being energy dependent, and at least partially reliant on the presence of intraluminal energy substrates. Specific intestinal immunity is governed by the gut-associated lymphoid tissue (GALT). The inductive sites in the Peyer’s patches provide an interface between antigen-presenting cells and circulating lymphocytes. Animal studies have demonstrated improved immunity in enterally fed groups.5
Patients may have inadequate appetite or gastrointestinal function to maintain optimal nutrition on oral intake alone. Enteral feeding has been used successfully to meet the nutritional needs of patients with a wide range of surgical diseases including cancer, inflammatory bowel disease, and pancreatic disorders. However, its use is contraindicated in cases of bowel obstruction, persistent intolerance, hemodynamic instability, major gastrointestinal bleeding, and inability to access the gastrointestinal tract safely.
Many enteral formulas now contain fiber, which may be soluble or insoluble. Insoluble fiber improves colonic function and bowel transit time, but there is no nutritional benefit or requirement. In contrast, soluble fiber binds to cholesterol and bile salts, and thus lowers serum cholesterol levels. Colonic bacteria digest soluble fiber and produce short-chain fatty acids that are utilized by the colonocyte as a fuel source.
Currently, there are over a hundred enteral products on the market. Most formulas have a caloric density of 1-2 kcal/ml, are lactose-free, and provide the recommended daily allowances of vitamins and minerals in less than 2 liters of formula per day. The majority of patients tolerate standard enteral formulas; however, elemental formulas may be necessary in patients with malabsorption. Recently, excellent results with improved immune function and surgical outcomes have been obtained with the preoperative administration of immunoenhancing formulas.6 Other disease specific formulations have been created for patients with liver disease, renal failure, pulmonary insufficiency, and diabetes. Formulas for patients with liver disease and encephalopathy contain a higher percentage of protein in the form of branched-chain amino acids with almost no aromatic amino acids. Renal failure formulas have very low levels of potassium and phosphorus. However, hepatic and renal formulas have a very low protein content, which must be considered. Patients with advanced pulmonary disease need to receive most of their calories as fat in order to decrease carbon dioxide production. Diabetic formulas also contain additional calories as fat, but also contain soluble fiber to decrease blood sugar levels. It is important to note that all of these specialty formulas are very expensive, and should only be used when a standard formulation with an appropriate nutrient profile has failed.
Preoperative enteral feedings have been demonstrated to decrease postoperative complication rates by 10%–15% of controls, but there is debate over the length of therapy needed to achieve this. The literature supports a course of enteral feedings for 5–20 days prior to surgery. Recently, it has been shown that there may be an advantage to utilizing immune-enhancing formulas, either alone as preoperative supplements, or in combination with postoperative support.6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree