96 Portal Hypertension
 Anatomy and Physiology of the Portal System
Anatomy and Physiology of the Portal System
The term portal system refers to a venous system that begins and ends in capillaries. The portal venous system commences in the capillaries of the intestine and ends in the hepatic sinusoids. The portal venous system drains blood from the gastrointestinal (GI) tract, pancreas, gallbladder, and spleen. The portal vein originates from the confluence of the splenic vein and the superior mesenteric vein. The inferior mesenteric vein and short gastric veins drain into the splenic vein. The superior mesenteric vein drains all the blood from the small bowel and the right colon, while the inferior mesenteric vein drains the blood from the remainder of the colon and most of the rectum. Flow in the portal vein is normally about 1 L/min (approximately 20% cardiac output) with a mean pressure of 7 mm Hg. Although the blood in the portal vein is the outflow from capillary beds and therefore has relatively low oxygen content, 70% of hepatic oxygenation is derived from portal flow. The blood flowing through the hepatic artery supplies the remainder of hepatic oxygen consumption and is the primary blood supply to the biliary tree. The portal vein carries a high concentration of nutrients and hormones, facilitating the liver’s central role in fat, carbohydrate, drug, and protein metabolism. Toxic substances are removed by hepatocytes, and bacteria (and bacterial products) are removed by Kupffer cells. Portal venous blood and hepatic arterial blood mix at the sinusoidal level, and there exists an adenosine-mediated local hepatic arterial autoregulatory “buffer response” that increases arterial inflow in response to low portal flow; however, total hepatic flow is not preserved when hepatic arterial flow is decreased. This buffer response is also dysregulated in sepsis.1
 Pathophysiology
Pathophysiology
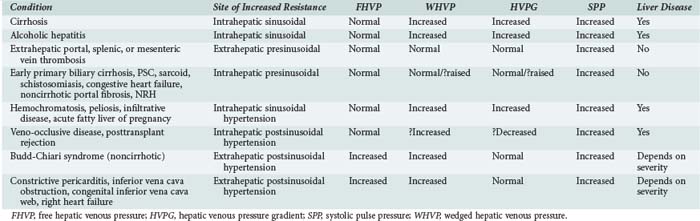
According to Ohm’s law (as applied to the cardiovascular system rather than an electrical circuit), the pressure within a vessel is determined by the flow of the blood in that vessel divided by the resistance. Apparent resistance depends upon a number of factors, including the length of the vessel, the radius of the vessel, and the viscosity of the blood. Since length and blood viscosity remain relatively constant, changes in radius are of paramount importance for determining changes in apparent resistance. An increase in blood flow in the portal vein and hepatic artery are important to the development of portal hypertension in some cases, but the increase in resistance seems to be the most important factor and is used to classify portal hypertension (PHT). The origin of PHT can be divided into cirrhotic and noncirrhotic and presinusoidal, sinusoidal, and postsinusoidal (Table 96-1). In response to PHT, vascular collaterals develop, and vascular resistance drops in the splanchnic bed, leading to the development of a hyperdynamic circulation. As a consequence, splanchnic and portal venous inflow increases, and PHT persists even with the development of vascular collaterals. As the pressure within the portal system continues to rise, portal blood flow decreases and hepatic perfusion deteriorates. The liver is deprived of portal blood, and this tends to accelerate the progression of liver disease. The hyperdynamic circulation and PHT also contribute to the development of portopulmonary syndrome (pulmonary hypertension and PHT), hepatopulmonary syndrome (hypoxia and intrapulmonary shunting in association with PHT), cirrhotic cardiomyopathy, ascites, and hepatorenal syndrome.
 Diagnosis of Portal Hypertension
Diagnosis of Portal Hypertension
PHT is defined as a portal pressure that is 5 mm Hg greater than the pressure measured in the inferior vena cava or a pressure of more than 15 mm Hg in the splenic vein or portal pressure measured at surgery. If the gradient is greater than 10 mm Hg, then clinically significant PHT is present. The direct consequences of PHT are formation of portosystemic collaterals and splenomegaly. Portosystemic collaterals can become clinically apparent as gastric or esophageal varices, umbilical vein recanalization, retroperitoneal collaterals, and/or rectal or ileostomy varices. The complications of PHT are variceal bleeding, ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, hyperdynamic circulation, and hypersplenism. Varices are rarely (maybe never) seen if the gradient is less than 10 mm Hg.2 Variceal bleeding is not observed if the pressure gradient is less than 12 mm Hg, and protection from variceal bleeding is gained if the pressure gradient can be manipulated to less than 12 mm Hg or a 20% reduction in pressure is achieved.3
Indirect measurements also can be used to assess the portal pressure gradient. This procedure involves measurement of the free and wedged hepatic venous pressure using catheterization of the right hepatic vein. Wedged hepatic venous pressure (measured using a balloon-tipped catheter) reflects the pressure in a static column of blood from the hepatic vein to the sinusoid. It is an assessment of sinusoidal pressure rather than portal venous pressure and therefore may underestimate the portal pressure gradient in disease states characterized by pre-sinusoidal hypertension (see Table 96-1). The free hepatic venous pressure is obtained with the catheter in the hepatic vein and gives an assessment of caval pressure. Free hepatic venous pressure is not elevated in patients with diseases characterized by pre-sinusoidal and sinusoidal PHT, but it is characteristically raised in post-hepatic (or extrahepatic postsinusoidal) etiologies. The gradient between the two measurements is called the hepatic venous pressure gradient and is the most commonly quoted parameter in the medical literature regarding management of PHT. Both the absolute value of hepatic venous pressure gradient and the change in hepatic venous pressure gradient with pharmacotherapy have prognostic significance related to the risk of variceal bleeding.4
 Complications of Portal Hypertension
Complications of Portal Hypertension
Varices
Two main mechanisms have been implicated in the pathogenesis of variceal hemorrhage in patients with established varices and PHT: erosions secondary to acid reflux and spontaneous rupture. Effects related to ascites and changes in plasma volume also have been implicated in the genesis of bleeding. Ascites may be a factor in variceal hemorrhage, because ascites can transmit intraabdominal pressure and thereby increase the pressure inside the esophageal varices. Some studies have shown a decrease up to 10% in the hepatic vein pressure gradient (HVPG) and a decrease in portal flow with paracentesis. Drainage of large volume of ascites can decrease intraabdominal pressure, leading to splanchnic dilation and increased blood flow against a fixed resistance in the liver.5 This circumstance would increase portal pressure and, hence, the risk of bleeding. The pressure inside the varices does seem to be affected by intraabdominal pressure, but whether this affects the risk of bleeding is not known. Erosions secondary to esophagitis also have been suggested as an important factor for the bleeding process.6,7 However, there was no evidence of acid reflux in patients studied with a pH electrode, and treatment with cimetidine did not affect the rates of rebleeding from varices.8
The pressure inside the varices is directly dependent on the portal pressure and also on the radius of the varix. The pressure within the varix is inversely proportional to wall thickness. Therefore, varices are more likely to bleed when they are larger and have a thinner wall. Large, thin-walled varices are generally located near the gastroesophageal junction where the veins are more superficial and less surrounded by other tissues. There is a relationship between the risk of bleeding and portal pressure. If the HVPG is greater than 20 mm Hg after an initial bleed, it is a poor prognostic sign, and there is a substantial risk of rebleeding and mortality.9 In a recent study, patients underwent portal pressure measurement after initial endoscopy and control of bleeding. Those whose pressure was above 20 mm Hg were randomized to early transjugular intrahepatic portosystemic shunt (TIPS) or conventional treatment. The group who underwent TIPS shunt insertion had an improved outcome compared to the standard-of-care high-risk group and similar to that of the low-risk group. Recent work has also suggested that portal pressure may be equally predicted by Child-Pugh score, and thus in this group of patients, consideration should be given to early TIPS shunt insertion.
It may be equally possible to measure variceal pressure at the time of endoscopic band ligation. This appears to be feasible and safe, although it remains at present an experimental method, and more studies are required.10
Portal hypertensive gastropathy is a complication of PHT that causes flow and pressure changes in the gastric mucosa. Mild or chronic bleeding is observed in 35% of the patients with mild gastropathy and 90% of those with severe gastropathy. Overt bleeding happens in 30% of those with mild and in 60% of those with severe gastropathy.11 The administration of propranolol decreases gastric mucosal blood flow and is effective in reducing bleeding in portal hypertensive gastropathy.12,13 TIPS and transplant are effective modes of treatment.
Diagnosis of Variceal Bleeding
When a patient with a possible hepatic disorder presents with hematemesis or melena, the most common cause of bleeding is from varices.14 Sometimes it is useful to insert a nasogastric tube followed by lavage of the stomach, both as a diagnostic tool and also to clear the gastric cavity before endoscopy. Since patients with PHT also can bleed from gastritis, esophagitis, Mallory-Weiss tears, and peptic ulcers, the most accurate method for the diagnosis of bleeding varices is upper endoscopy; accuracy exceeds 90%. Frequently one or two doses of 250 mg of erythromycin are administered before the procedure to help clear the stomach before the procedure.
Acute Variceal Hemorrhage
Because of the high risk of infection in this population of patients, it is necessary to give prophylactic antibiotics. In a recent meta-analysis, treatment with antibiotics was associated with an increase in hospital survival and decrease in infection. The risk of rebleeding also was lower in patients receiving antibiotics.14,15 Oral norfloxacin, 400 mg twice a day; intravenous (IV) ciprofloxacin, 400 mg twice a day; or levofloxacin, 500 mg twice a day are the recommended antibiotics in 5-day courses.
The lack of tachycardia or hypotension in these patients is not indicative of stability, because up to 25% of blood volume may be lost without any hemodynamic changes.16 Blood volume should be restored, and coagulation factor support in the form of fresh frozen plasma and platelets may be required.
 Treatment
Treatment
Pharmacotherapy
Terlipressin (Glypressin) is a prodrug of vasopressin that has some intrinsic activity. It acts on vasopressin-1 receptors within arteriolar smooth muscle and induces vasoconstriction via phospholipase C–dependent signaling.17 Treatment with terlipressin results in splanchnic vasoconstriction and decreases splanchnic inflow, thereby reducing portal pressure. Terlipressin also reduces collateral blood flow and variceal pressure.18
Compared with vasopressin, terlipressin is associated with a lower incidence of systemic ischemic events, and unlike vasopressin, terlipressin can be used safely without coadministration of nitroglycerin or other organic nitrates. Terlipressin has a longer half-life than vasopressin and can be administered intermittently. A dose of 2 mg IV given four times daily is as effective as endoscopic sclerotherapy for achieving initial control of variceal bleeding and preventing early rebleeding.19 Terlipressin has been shown to decrease mortality and length of stay when administered to a high-risk population of patients presenting with acute upper GI hemorrhage.20
Terlipressin is well tolerated and has few side effects and may represent first-line treatment in acute hemorrhage until endoscopy can be performed in a controlled environment. In a recent meta-analysis, terlipressin was more effective than placebo but less effective than octreotide for controlling bleeding.21 Despite these findings, a recent study showed equivalence between terlipressin and octreotide.22,23
The duration of treatment should be governed by the clinical situation. After 48 hours of therapy, however, the dose should be tapered (initially halved), seeking to achieve a course of therapy lasting 6 to 7 days. A recent study compared endoscopic banding therapy and banding in addition to 5 days of terlipressin.24 Outcome was improved in the cohort that received combined therapy.
Treatment with somatostatin also may be considered. The dose of somatostatin is 250 µg as a bolus followed by 250 µg every hour as an infusion. The efficacy of somatostatin in the control of bleeding is not totally clear. In a recent meta-analysis of studies of somatostatin compared to control or no treatment, the use of somatostatin was associated with initial hemostasis but not with a decrease in mortality or rebleeding.25 The treatment effect of somatostatin amounted to lowering the transfusion requirement by 0.5 units of blood per patient. In view of these findings, somatostatin cannot be recommended as the first-line agent for the control of variceal bleeding.
The somatostatin analogue, octreotide, may act by blocking the acute rise in portal pressure associated with fluid resuscitation in the face of GI hemorrhage. Its use is associated with improved outcome after therapeutic endoscopy. Octreotide is a somatostatin analog that has much longer half-life and therefore can be given as a bolus or infusion. Octreotide acts by blocking the vasodilatory effects of glucagon and vasoactive intestinal peptide. The side-effect profile for octreotide is more favorable than the side-effect profiles for terlipressin or vasopressin. In a recent meta-analysis, octreotide was more effective than no treatment or vasopressin/terlipressin.18
In a recent study, vapreotide was given for 5 days to patients acutely bleeding from varices.26,27 A decrease in the number of bleedings during the index endoscopy and in the next 5 days was observed.
Therapeutic Endoscopy
The timing of endoscopy has recently been addressed; one study suggested that the determinants of outcome following acute variceal hemorrhage were “door-to-needle time” (threshold value: 15 hours) and Model for End-stage Liver Disease (MELD) score.28 A similar study from a Canadian group did not show any effect of door-to-needle time.29 However, in this study, hemodynamically unstable patients were excluded and underwent endoscopy within 4 hours. Determinants of outcome were infection on admission, albumin level, and MELD score.
Sclerotherapy
In the sclerotherapy approach, a sclerosant is injected directly into the varix. A variety of sclerosants are in use, but ethanolamine and sodium tetradecyl sulfate are the most common. The immediate effect of controlling bleeding is probably due to edema caused by the injection of the sclerosant; thrombosis occurs later. Injection sclerotherapy can be accompanied by complications (Table 96-2). The rate of mortality related to severe complications is approximately 15%. The most common long-term complication is esophageal stricture.
TABLE96-2 Complications of Endoscopic Sclerosant Therapy
| Site | Complication |
|---|---|
| Local | Ulcers |
| Bleeding | |
| Stricture | |
| Esophageal dysmotility | |
| Regional | Perforation |
| Mediastinitis | |
| Pleural effusion | |
| Systemic | Sepsis |
| Aspiration |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree