b. Blood volume does not return to the prepregnancy level for greater than 6 weeks’ postpartum.
2. Cardiac output4 (Table 1.2)
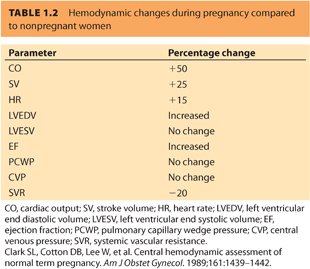
a. Cardiac output (CO) begins to increase at 10 weeks’ gestation, peaking at 40% to 50% of baseline at 32 weeks’ gestation.
b. The increase in CO is a result of combined increases in stroke volume (SV) and heart rate (HR) during pregnancy. HR peaks at 10 to 20 beats per minute (bpm) above the prepregnancy rate at term. During labor, SV increases dramatically, whereas HR increases slightly, leading to up to an additional 40% increase in CO by the second stage of labor.
c. Immediately postpartum, it is possible for maternal CO to rise 75% above predelivery values.
d. CO returns to a prepregnancy level around 2 weeks postpartum.
3. Systemic vascular resistance. Systemic vascular resistance (SVR) is decreased through a number of mechanisms5:
a. The low-resistance placental circulation is essentially in parallel with the systemic circulation. Because the sum of two resistances in parallel is less than either alone, the placental bed serves to decrease afterload.
b. Progesterone contributes to vasodilation through its effect on vascular smooth muscle.
c. Plasma levels of prostacyclin, a potent vasodilator, are increased in pregnancy.
d. Blood viscosity is a significant determinant of afterload. The dilutional anemia of pregnancy improves the rheology of blood,1 decreasing afterload.
4. Myocardial contractility
a. Left ventricular (LV) end diastolic volume increases during pregnancy but LV end systolic volume remains the same, which results in an increase in ejection fraction.6
b. Left ventricular hypertrophy (LVH) occurs progressively throughout pregnancy, with a 23% increase in mass from the first to third trimesters.7
c. Although an increase in myocardial contractility is suggested by an increase in the velocity of LV circumferential fiber shortening, this can be explained on the basis of an increase in HR and decrease in SVR. Intrinsic contractility as measured by left ventricular stroke work index (LVSWI) is unchanged.
d. HR increases steadily throughout pregnancy, peaking at 10 to 20 bpm above baseline at term. Further increases are seen as a result of the pain and stress of labor.
B. Electrocardiogram changes and rhythm disturbances
1. The electrocardiogram (ECG) at term pregnancy may show changes that result from a leftward shift of the heart because of elevation of the diaphragm by the gravid uterus. The following are all potentially normal ECG findings in pregnancy8:
a. Shift in QRS axis in any direction
b. Small rightward deviation of average mean QRS axis in the first trimester
c. Small leftward deviation of mean QRS axis due to progressive elevation of left hemidiaphragm in the third trimester
d. Lead III often shows small Q-T wave inversions.
e. Transient ST-T wave changes are common.
2. Functional flow murmurs are common during the hyperdynamic state of pregnancy,9 and there may be a predisposition to tachydysrhythmias (especially supraventricular). The most common dysrhythmias in pregnancy are premature ectopic atrial and ventricular depolarizations and sinus tachycardia.10 Pregnant women become more aware of their heartbeat, changes in HR, and skipped beats. Mechanisms for pregnancy-induced dysrhythmias include:
a. Changes in cardiac ion channel conduction
b. Increase in cardiac size (atrial stretch, increased end diastolic volume, LVH)
c. Changes in autonomic tone
d. Hormonal fluxes
C. Aortocaval compression
1. Compression of the inferior vena cava against the vertebral column by the enlarged uterus (significant once the uterus moves out of the pelvis between 16 and 20 weeks’ gestation) when in a supine position will lead to a reduction in venous return. Although this is partially compensated by collateral blood flow, for example, through the azygos system, the net result is a decrease in venous return to the heart and subsequently CO is decreased.
2. Compression of the aorta by the enlarged uterus may increase measured blood pressure in the arm, by a mechanism analogous to an aortic cross clamp. Because the hypogastric artery, of which the uterine artery is a branch, arises distal to the point of compression, uteroplacental perfusion may be decreased in spite of an apparently increased systemic blood pressure as described earlier. Significant aortoiliac artery compression by the gravid uterus is seen in 15% to 20% of pregnant women. In many nonlaboring patients, this is asymptomatic.
3. A recent study evaluated the degree of tilt necessary to minimize aortocaval compression in term, nonlaboring patients prior to cesarean delivery (CD). CO and pulse pressure were highest at 15 degrees of left tilt, equal to full 90-degree left lateral position. This was significantly higher than at 0 and 7.5 degrees of left tilt, suggesting that 15 degrees is sufficient to restore CO.11
4. As a result, the supine position should be avoided in all pregnant patients at 20 weeks’ gestation or greater, and especially in all term parturients. This applies even more following placement of a neuraxial anesthetic.
CLINICAL PEARLThe enlarged uterus causes aortocaval compression in the supine position and may lead to decreased venous return, decreased CO, and severe hypotension. When the supine position is required, the patient must be placed with at least a 15-degree left lateral tilt. One of the most effective ways to assess for effective left uterine displacement is to visualize the displacement of the uterus from the perspective of the head of the patient’s bed.
II.Respiratory system
A. Arterial blood gases
1. Progesterone sensitizes central respiratory centers, increasing the ventilatory response to CO2.12 Both tidal volume (major contributor) and respiratory rate (negligible to 1 to 2 bpm) are increased and contribute to pregnancy-induced increased minute ventilation. A recent study has shown that the hyperventilation of human pregnancy is the result of pregnancy-induced changes in wakefulness and central chemoreflex drives for breathing, acid–base balance, metabolic rate, and cerebral blood flow.13 This explains why normal PaCO2 in a pregnant patient is 30 to 32 mm Hg (Table 1.3). Although there is increased urinary excretion of bicarbonate (normal pregnant level 20 mm Hg), pH is partially corrected; normal pH is 7.41 to 7.44.14

2. Hyperventilation causes decreased alveolar CO2, which, by the alveolar gas equation, leads to an increase in PaO2 (normal 103 to 107 mm Hg).
B. Lung volumes and capacities and respiratory mechanics8 (Table 1.4)

1. Anatomic changes listed below contribute to altered ventilatory mechanics over the course of a normal pregnancy.15
a. Elevation of the diaphragm occurs due to increased intraabdominal volume with the growing uterus. Diaphragmatic excursion increases relative to the nonpregnant state.
b. An increase in the anterior–posterior diameter of the thoracic cage causes decreased chest wall excursion.
C. Mechanisms of hypoxemia in pregnancy16
1. Oxygen consumption (Table 1.5)

a. The high metabolic demands of the enlarged uterus, placenta, and the fetus cause oxygen consumption to increase throughout pregnancy, increasing 40% to 60% above prepregnancy levels at term.17
b. Oxygen desaturation occurs much more rapidly during a period of apnea, for example, during anesthetic induction or eclamptic seizures. This effect is accentuated by changes in pulmonary volumes (q.v.). In one study, reduced apnea tolerance in pregnancy was demonstrated by simulating the physiologic changes of rapid sequence induction.18 The authors found that after 99% denitrogenation, the time taken to decrease to a SaO2 <90% was 4 minutes in pregnant subjects and 7 minutes 25 seconds in nonpregnant subjects. In addition, the time taken for SaO2 to fall to 40% from 90% was 35 seconds in pregnant subjects and 45 seconds in nonpregnant subjects. During pregnancy, the hypoxic ventilatory response is increased due to elevations in estrogen and progesterone.
c. Fetal hemoglobin has a P50 of approximately 18 mm Hg, providing very effective loading of oxygen (and unloading of maternal hemoglobin) in the uteroplacental bed. In addition, fetal hemoglobin does not interact with 2,3-diphosphoglycerate, which also favors loading of oxygen molecules from maternal hemoglobin. Thus, the fetus effectively extracts the maximum amount of oxygen possible from maternal blood.
2. Decreased functional residual capacity. Elevation of the diaphragm during pregnancy leads to increased basilar alveolar atelectasis. Functional residual capacity (FRC) essentially represents the stores of oxygen available during a period of apnea; therefore, a decrease in FRC shortens the time to development of hypoxemia.
CLINICAL PEARLParturients are susceptible to rapid decreases in oxygen saturation with periods of apnea due to decreases in FRC and increased oxygen consumption. Preoxygenation of the parturient prior to general anesthesia and endotracheal intubation is essential and will help alleviate severe arterial oxygen desaturation.
3. Compared to sitting or standing, FRC is significantly lower in the supine position due to further cephalad movement of the diaphragm and increased posterior basilar atelectasis. In the supine position, FRC exceeds closing capacity. This leads to small airway closure, an increase in ventilation/perfusion (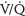 ) mismatch, and decreased O2 saturation.
) mismatch, and decreased O2 saturation.
4. Decreased CO in the supine position will cause decreased mixed venous saturation and therefore decreased arterial O2 saturation. Appropriate left uterine displacement will help maintain adequate venous return and subsequent CO.
D. Upper airway changes (see Section IX: Anesthetic considerations)
E. Respiratory consequences of uncontrolled maternal pain
1. Uncontrolled maternal pain causes exaggerated hyperventilation during contractions. Minute ventilation can increase by as much as 140% above prepregnant values in the first stage of an unmedicated labor and 200% in the second stage.19,20 As a result, maternal PaCO2 can fall to 10 to 15 mm Hg. This extreme degree of hypocarbia may cause subsequent maternal hypoventilation.
2. At the same time, oxygen consumption is increased (i.e., a doubling of ventilation by the parturient can increase oxygen consumption by as much as 50%).19 As a result, significant hypoxemia can occur between contractions. Maternal blood lactate increases, indicating that aerobic oxygen requirements exceed oxygen consumption during labor. Although hypoxic ventilatory drive is increased during pregnancy, it may not be possible to match oxygen consumption without supplemental oxygen.
F. Oxygen delivery
1. The mild increase in dissolved oxygen in maternal blood during pregnancy does not improve oxygen delivery to the fetus.
2. However, oxygen delivery to the fetus is improved by a shift of the maternal oxygen dissociation curve to the right.21 The maternal P50 increases from 26 to 30 mm Hg at term.
3. As mentioned earlier, fetal ability to extract oxygen from maternal hemoglobin is augmented by the higher oxygen affinity of fetal hemoglobin, which has a P50 of 18 mm Hg.
III.Hematologic system
Normal pregnancy is associated with multiple changes in the hematologic system (Table 1.6) and it is important that the anesthesiologist be aware of these changes in order to determine what is normal and what is abnormal when reviewing laboratory results in the pregnant woman.

A. Dilutional anemia
1. Red cell mass is increased during pregnancy. Plasma volume increases to a greater extent, however, producing the so-called dilutional anemia of pregnancy.
2. In the absence of dietary iron supplementation, hemoglobin levels of 9 to 10 g per dL are typical.3
3. Hemoglobin levels of >13 g per dL suggest hemoconcentration and may be a sign of preeclampsia.
B. Platelet count and function
1. In most parturients, there is either a moderate decrease in platelet count or no change. Some studies have indicated that there is evidence of increased platelet consumption during pregnancy. However, other studies have shown increases in platelet production and turnover.
2. Gestational thrombocytopenia (platelet count 90 to 100 × 109 per L) is a physiologic condition that occurs in a small percentage of parturients.22 It resolves spontaneously postpartum and is not associated with abnormal platelet function or clinical bleeding.
C. Coagulation factors
1. Normal pregnancy is associated with profound alterations in the coagulation and fibrinolytic systems (Table 1.6).
2. Although physiologic procoagulant changes serve to minimize intrapartum blood loss, they also increase the risk of thromboembolism during pregnancy and the postpartum period sixfold.23
3. The net effect of these changes is to increase the efficiency of clotting and to impair fibrinolysis.24
4. Fibrinogen levels increase throughout pregnancy and at term are often >400 mg per dL. If fibrinogen is less than 200 to 250 mg per dL, a pathologic process should be suspected.
5. This hypercoagulable state cannot be detected by conventional tests such as the prothrombin time and activated partial thromboplastin time because results of these tests remain only slightly below or in the normal range. However, calibrated automated thrombography (CAT) is able to demonstrate an increase in endogenous thrombin potential throughout pregnancy.25 The CAT test measures protein S levels and activity, which are reduced significantly during pregnancy, and is able also to indicate increases in plasminogen activator inhibitor-1, thrombin–antithrombin complex, and tissue factor pathway inhibitor during pregnancy. Antithrombin levels and protein C levels remain stable throughout pregnancy. It is unknown whether these observed changes correlate with thromboembolic disease.
CLINICAL PEARLPregnancy is associated with hyperfibrinogenemia. Decreases in serum fibrinogen, especially below 200 mg per dL, are associated with severe postpartum hemorrhage. In this situation, one should consider early replacement of clotting factors by administration of fresh frozen plasma (FFP) or cryoprecipitate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







