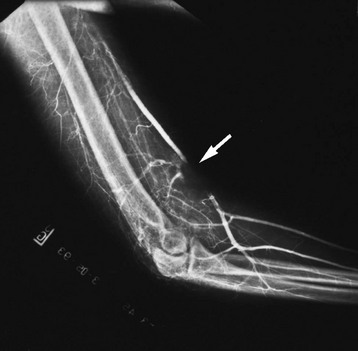
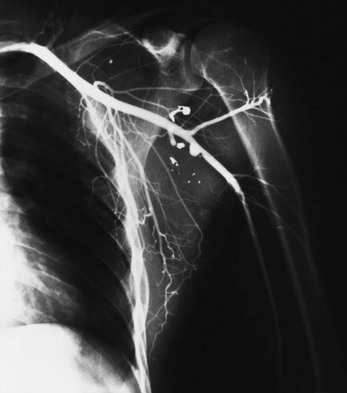
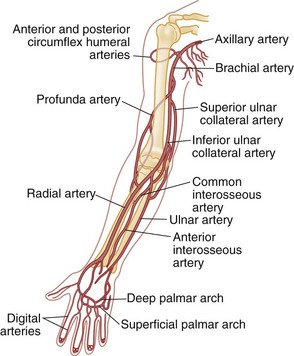
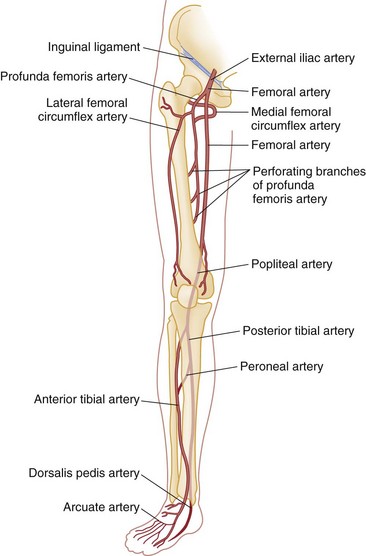
Chapter 48 Injury to the major peripheral arteries or veins is often life-threatening but invariably poses a threat to the viability of the affected limb. Historically, because of rapid blood loss, injury to major vessels was often quickly fatal in the field. Most patients who survived to reach a hospital had relatively minor vascular injuries. However, with the advent of modern emergency medical services systems with advanced extrication methods and rapid transport, more patients with major vascular injury reach the hospital alive.1 In addition, the incidence of penetrating civilian injuries from interpersonal violence and blunt injuries from motor vehicle–related trauma in the United States has increased dramatically during the past 50 years. Consequently, emergency physicians are frequently confronted with critically ill patients harboring overt or occult vascular injuries. Management of vascular injuries has evolved with advances in diagnostic methods and surgical techniques. Treatment of vascular injuries before and during World War II was simple ligation of the peripheral artery or vein involved. This approach resulted in limb amputation rates ranging from 40% for axillary artery injuries to 72% for popliteal artery injuries. During the Korean War, routine attempts to repair injured arteries decreased the amputation rate for popliteal injuries to 32%.2,3 During the Vietnam War, repair of penetrating axillary and popliteal artery injuries with routine angiography and improved surgical techniques resulted in decreases in the amputation rate to as low as 5 and 15%, respectively, which approach the current rates of amputation for civilian injuries.2,3 Owing to the nature of the munitions and the extensive use of body armor in the Iraq and Afghanistan wars, the proportion of severe wounds of the extremities has increased. The amputation rates thus far are 5% for upper extremity and 21% for lower extremity vascular injuries.2 However, extrapolation of high-velocity military wound data to low-velocity civilian gunshot wounds may not be valid, and even lower rates might be expected with civilian wounds. Throughout the world, the causes of peripheral vascular injuries are divided almost equally between blunt and penetrating mechanisms. In the United States, 56 to 90% of these injuries are a result of penetrating wounds, mainly because of the high rate of penetrating trauma in inner-city urban areas.4–7 Although the incidence of low-velocity gunshot wounds has decreased during the past decade, gunshot wounds remain the second leading cause of death in the 15- to 34-year-old age group in the United States.8 Major venous injuries are present in 13 to 51% of all cases, but more than 80% of these are associated with arterial injury as well.9 Approximately 90% of patients with vascular injury are male, and most are younger than age 40 years.5 Because of the increased use of percutaneous endovascular diagnostic and therapeutic procedures, the incidence of iatrogenic vascular injuries has increased and accounts for up to one third of all cases in some series.10,11 Blunt injury involves avulsion forces that can stretch vessels beyond their capacity or direct crushing injury that disrupts the vessel wall. Fracture fragments resulting from blunt extremity trauma can lacerate or entrap vessels. Vascular injury can range from small intimal tears to complete avulsion of arteries and nerves. Open avulsion injury of a limb is particularly severe because the skin is the final structure to tear, and once such tearing has occurred, it is inevitable that vessels and nerves will be torn as well. Vascular injury also should be suspected in patients with massive soft tissue avulsion or crush injury, displaced long bone fractures, electrical or lightning injuries, and severe burns, as well as in those with compartment syndrome from trauma or prolonged immobilization as a result of stroke, coma, drug overdose, or other causes.12–15 Bites that are inflicted by large animals, such as dogs used by law enforcement, are particularly prone to arterial injury and wound complications.12,13 Collateral circulation may continue to perfuse the limb adequately, but injuries that occur proximal to the collateral branch point or that involve both the main trunk and collateral branches will preclude adequate flow. When no specific measures are taken to cool the limb, it is said that the limb is undergoing “warm ischemia” at room temperature. Although the time in individuals may vary, 6 hours of complete warm ischemia is generally considered the point at which irreversible nerve and muscle damage begins to occur. After 6 hours of warm ischemia, 10% of patients will have irreversible damage; by 12 hours, 90% will have irreversible damage. Artificially cooling the limb to just higher than freezing temperature (cold ischemia) will reduce the metabolic demands of unperfused tissues and greatly prolong the tissue’s tolerance of ischemia to 24 hours or more. Animal studies suggest that hyperbaric therapy may be useful in repaired limbs that have undergone prolonged warm ischemia.16 Transection.: The most common vascular injury is complete transection in which distal flow is effectively eliminated. Cleanly transected arteries will often retract and undergo spasm so that blood loss is minimized. With longitudinal arterial lacerations and venous injuries, blood loss cannot be limited by this means, and such injuries tend to result in greater blood loss. Pulsatile bleeding may lead to exsanguinating hemorrhage and shock. Thrombosis.: Intraluminal thrombosis (Fig. 48-1) may occur in an injured artery acutely (within 24 hours) or may be delayed for many months. Acute thrombosis is initiated by stasis resulting from compression of the artery or from a disruption in the intima of an artery that becomes a nidus for thrombus formation. As the thrombus propagates, complete occlusion of the vessel can occur. Delayed thrombosis can occur months to years after injury if the injured vessel heals with stricture formation and decreased blood flow distally, followed by stasis and clot formation. Reversible Arterial Spasm.: The precise cause and incidence of significant reversible arterial spasm after trauma are unknown. In the case of arterial transection, arterial spasm is beneficial and limits hemorrhage. In other cases, however, the segmental arterial spasm occurs at some distance from the site of traumatic injury and can produce severe distal ischemia. Arterial spasm is particularly common in children. The spasm usually reverses with conservative treatment (topical warm saline or topical nitroglycerin paste), but prolonged spasm may require infusion of vasodilators such as nitroglycerin, calcium channel blockers, alpha-blockers, nitroprusside, specific prostaglandin inhibitors, or warm saline.17 In many series, segmental arterial spasm is the most common arteriographic finding. However, it should never be assumed on clinical grounds that symptoms of ischemia are a result of arterial spasm; that diagnosis is based on arteriographic results only. Intimal Flap.: An intimal flap occurs when there is a break in the intima of a vessel, generally from excessive stretch or concussive forces. Although flow is not altered by small flaps and the associated soft tissue wounds often appear benign initially, these intimal flaps may become a nidus for thrombosis that can occur hours to months after the initial injury. However, most intimal flaps heal spontaneously, and asymptomatic injuries that do not disrupt perfusion of the limb can be treated conservatively with antiplatelet agents such as clopidogrel. Pseudoaneurysm.: A true aneurysm contains all three layers of the vessel wall (intima, media, and adventitia) and rarely is caused by trauma. A pseudoaneurysm is formed following a tear in a vessel wherein the hemorrhage is contained by surrounding fascia and the resulting hematoma is gradually encased by a capsule of fibrous tissue, analogous in consistency to the adventitia of a normal vessel (Fig. 48-2). Because it is relatively thin walled, rupture of a pseudoaneurysm is a distinct possibility. In addition, because its diameter inevitably expands under arterial pressure over days to months, compression of adjacent tissue may result in neuropathy, venous obstruction with resultant peripheral edema and venous thrombosis, and even erosion into adjacent bone.18 The cavity of a pseudoaneurysm is in direct communication with the lumen of the vessel, so embolization of mural clots may produce distal arterial occlusion. Patients with pseudoaneurysm are commonly seen months to years later with symptoms of compression neuropathy or peripheral arterial embolism or for investigation of a soft tissue “tumor” that represents the growing aneurysm. Arteriovenous Fistula.: An AVF is formed when both the artery and an adjacent vein are injured. Higher-pressure arterial flow is directed into the lower-pressure vein, thereby diverting the blood supply to distal tissues and engorging the distal veins. Because the aperture of the fistula is often relatively narrow and thus results in turbulent flow, a bruit and palpable thrill are common diagnostic findings. Symptoms are primarily those of distal ischemia, but rarely, high-output congestive heart failure may occur when large central vessels are involved. Symptoms are often delayed for months because it takes time for the fistula to mature. Compartment Syndrome.: Compartment syndrome is most common after crush injury or a long bone fracture but may also be seen after reperfusion of an ischemic limb. Initially, blood flow is diminished and the injury can be considered nonocclusive. Progressive edema elevates tissue pressure above capillary pressure, thus ending arterial perfusion and initiating a cascade of events that results in compartment syndrome. The risk for this complication is increased when ischemia time is prolonged; in the presence of combined arterial and venous injury; after ligation or repair of a major artery or vein; or in the presence of significant soft tissue injury, frequently concomitant with a long bone fracture.5 Smaller caliber vessels are compressed first, whereas larger vessels remain relatively patent and compartment pressure rarely exceeds arterial pressure, so pulses may be palpable until very late in the course. If the condition is allowed to progress, however, all blood flow may end and the injury is then an occlusive one. After restoration of arterial flow to a previously ischemic limb, a cascade of reperfusion injury has been identified that results from release of oxygen free radicals, lipid peroxidation, and influx of intracellular calcium. These mediators give rise to progressive cellular damage, edema, and necrosis, thereby propagating the vicious cycle that increases compartment pressure.19 Consequently, frequent reexamination of the limb is indicated to assess compartment pressure after arterial repair or in the high-risk circumstances listed earlier. Detection and treatment of vascular injuries takes place within the context of the overall resuscitation of the patient according to established principles of trauma care.20 If the source of bleeding is readily identifiable, it is compressed with digital pressure. While control of active bleeding is being achieved in this manner, detection and treatment of other life-threatening injuries proceed concurrently. Peripheral vascular injury can occur coincident with other life-threatening trauma, which may take higher priority in resuscitating the patient. In other cases, peripheral vascular injury may be the most serious or only injury, and evaluation and management of this type of injury can proceed directly. Despite rapid transport to a hospital through a modern emergency medical service, injury to large central arteries and veins is still often fatal, and many of these deaths occur before medical contact. Patients who survive to reach the hospital may have obvious exsanguinating hemorrhage or only very subtle signs of vascular injury. Many patients have no evidence of injury but are considered at risk for vascular injury because of penetrating wounds that traverse the course of major neurovascular bundles or because they have sustained high-risk injuries such as posterior knee dislocation. Patients who remain hypotensive after an initial fluid challenge may harbor an occult vascular injury if no other cause is found. In addition, patients with symptoms of intermittent claudication or with unexplained peripheral embolization and a history of previous trauma to the limb should be suspected of having occult arterial injury. Many patients have the classic “hard” findings of arterial injury, including pulsatile bleeding, loss of distal pulses, an audible bruit or palpable thrill indicative of an AVF, or an expanding or pulsatile hematoma.21 In addition, pallor or cyanosis and decreased temperature are common in a poorly perfused extremity, and massive distention of distal superficial veins may indicate an AVF as arterial flow is directed into distensible veins. The incidence of arterial injury in patients with any hard finding is consistently greater than 90%,22,23 and the presence of these findings requires further investigation by emergency angiography or, more commonly, immediate surgical intervention, depending on the duration of warm ischemia and the overall status of the patient. An additional group of patients have “soft findings” of vascular injury, including a palpable but diminished pulse in comparison with the uninjured extremity, isolated peripheral nerve injury, history of severe hemorrhage in the field, unexplained hypotension, or a large nonpulsatile hematoma.21–24 The significance of prolonged capillary refill is controversial; some experts find it to be a reliable sign of vascular injury (when combined with a pulse deficit) and consider delayed capillary refill to be a valid “soft sign” of vascular injury. Others have found this sign to be a nonspecific and unreliable predictor of arterial injury.5 Delayed capillary refill by itself is insufficient to diagnose arterial injury, but in combination with other physical signs it supports the diagnosis. Isolated penetrating injury to a peripheral nerve is commonly associated with vascular injury because of the close proximity of these structures within the neurovascular bundles. Vascular injury occurs in 8 to 45% of cases of penetrating peripheral nerve injury.25,26 Conversely, vascular injuries have associated peripheral nerve injury in almost half of cases. It is sometimes difficult to distinguish whether the pain, paresthesias, or paralysis is caused by a primary nerve injury, an associated vascular injury causing compression of the nerve, or compartment syndrome. In general, primary nerve injury occurs immediately at the time of injury, whereas vascular neuropathy occurs over minutes to hours after the injury. Up to 35% of patients with “soft” findings of vascular injury have positive angiographic studies, although only a small proportion of these injuries require emergency repair.21,26,27 The proximity of a penetrating wound to a neurovascular bundle is defined imprecisely. Various definitions include 1 cm, 1 inch, or 5 cm as constituting “proximity.” Certainly, penetrating wounds that occur within 1 cm of a major neurovascular bundle or whose presumed trajectory has crossed such a bundle (“proximity wounds”) are more likely to produce an occult vascular injury. Major neurovascular bundles include large limb arteries proximal to critical branch points, such as the axillary, brachial, common femoral, and popliteal arteries (Figs. 48-3 and 48-4).28 In addition, a small minority of patients with high-risk injuries, such as bites from large dogs or other animals, shotgun wounds, severely displaced fractures, crush injuries, or major joint dislocations (especially knee dislocation), will initially have occult vascular injury that may not be detected on physical examination.29 The risk of missing such injuries is that the traditional 6-hour window of warm ischemia time will be exceeded or the patient will experience delayed complications resulting in loss of the limb. For example, patients with intimal flaps may be completely asymptomatic initially but can subsequently develop arterial thrombosis. Similarly, pseudoaneurysms progressively enlarge to produce compression of adjacent structures but may be very small and undetectable on initial physical examination. Consequently, some centers routinely perform radiographic confirmation of arterial patency in these cases, although in most large-volume trauma centers current practice does not consider proximity alone as an indication for imaging. Surprisingly, in this era of increased reliance on technology, meticulous physical examination in combination with comparison of blood pressures in the affected and unaffected limbs has reemerged as the mainstay of diagnosis of vascular injury.22 Physical examination is directed at discovering evidence of local wound complications and distal ischemia suggestive of vascular injury. Palpation of pulses in the affected extremities is the initial step. A comparison of the strength and quality of the pulses between the injured limb and its uninjured counterpart is then made. Isolated detection of a diminished pulse distal to the site of injury is a finding that merits further investigation rather than immediate surgery because palpation of pulses is a relatively inaccurate means of predicting arterial injury. False-positive findings of a pulse deficit may occur because of shock, in which all pulses are diminished; congenital absence of a pulse in one extremity; preexisting vascular disease; or arterial spasm or compression. A false-positive finding of a pulse deficit occurs in 10 to 27% of cases.5,22,23 False-negative findings can occur with transmission of the pulse through a “soft clot,” past an intimal flap, or through collateral circulation. Distal pulses can persist in 6 to 42% of patients despite significant arterial injury.30 Compression of an artery by casts, splints, or dressings may produce a pulseless extremity, and these should be removed if evidence of ischemia occurs. Finally, although the pulse may be absent, the limb may be well perfused by collateral arterial supply, thus making immediate repair of the arterial injury less compelling. Simultaneous palpation of the injured and unaffected limbs can detect relatively small differences in skin temperature that may suggest hypoperfusion. Testing two-point discrimination on the injured and unaffected limbs is similarly an effective means of detecting sensory deficits. Auscultation over the site of injury is an often-ignored examination that may reveal a bruit suggestive of an AVF. A bruit is audible in more than half of patients with an AVF.31 Repeated examination of the hematoma adjacent to the wound is indicated during the first 24 hours to determine whether it is expanding or pulsatile. Despite the limitations just noted, reliance on the history and physical examination to triage patients into immediate surgery, imaging studies, and observation groups has been found to be relatively dependable, with a sensitivity of 92% and a specificity of 95%.22 However, studies of military casualties injured by blast injury or high-velocity gunshot wounds have found physical examination to be less reliable than in studies of civilian casualties.32 Plain radiographs of the affected extremity are indicated to detect fractures, joint penetration, and foreign bodies. With gunshot wounds, the sum of the number of intact bullets seen on radiographs and the number of entrance and exit wounds in the body should be an even number. Failure to locate a bullet can result in unexpected complications. Rarely, bullets or shotgun pellets can migrate distally and produce vascular occlusion or migrate proximally through the venous system to the heart. These emboli are readily detected on plain radiographs if the search is vigilant.33,34 Lead bullets retained within a synovial joint can result in systemic absorption and elevated lead levels and should be removed electively.35–41 Several relatively simple noninvasive maneuvers can be performed at the bedside to elicit evidence of arterial injury. The use of pulse oximetry has been suggested as a means of identifying limb ischemia after trauma, but it has been found to be relatively insensitive for this purpose. Clearly, in the absence of a pulse, no reading can be obtained. As the technology of transcutaneous measurement of physiologic indices advances, measurement of tissue oxygenation by near-infrared spectroscopy (NIRS) to quantify muscle oxyhemoglobin may prove more useful in detecting vascular injury because these devices are portable, noninvasive, and simple to use and appear to provide more accurate information than pulse oximetry.42–47 Thus far, however, small clinical studies have found contradictory results in use of NIRS for this indication.48,49 In an effort to improve on the accuracy of physical examination without relying on expensive and invasive tests such as arteriography, the use of blood pressure in the injured versus the uninjured extremity (arterial pressure index [API]) or the blood pressure in an injured leg at the ankle compared with brachial artery pressure (ankle-brachial index [ABI]) was developed and validated in numerous studies as an accurate means of detecting vascular injury. Systolic pressure is measured by inflating a standard blood pressure cuff proximal to the injury and recording hand-held Doppler systolic pressure distal to the injury. The process is repeated on the uninjured limb, and a ratio of injured to uninjured systolic pressure is calculated (API). In general, a ratio less than 0.90 is considered abnormal and is an indication for further investigation. In several studies, an API less than 0.90 yielded a sensitivity of 95%, specificity of 97%, positive predictive value of 100%, and negative predictive value of 95%.5,21,50–52 However, a few studies have found API to be less accurate, including one in which a cutoff of 0.90 resulted in a false-negative rate of nearly 40%; nevertheless, the use of an API ratio less than 0.90 can eliminate a large number of unnecessary angiograms for proximity wounds and increase the diagnostic efficiency of angiography or computed tomographic angiography (CTA) by limiting its use to high-yield cases. Patients with an API of 0.90 to 0.99 merit observation for 12 to 24 hours for repeated physical examination and API measurements to detect evolving injury. Patients with normal physical examination findings and a completely normal (>0.99) ABI can be safely discharged from the emergency department provided there are no other injuries requiring admission.52 Exclusive reliance on API to screen for arterial injury has significant limitations. Comparisons cannot be made when both limbs are injured or when severe soft tissue mangling precludes placement of a blood pressure tourniquet or location of the artery to be measured with the Doppler unit.21 As with physical examination, the sensitivity of API is limited when an intimal flap allows near-normal flow or when collateral circulation is sufficient to produce near-normal systolic pressure, as in proximal injuries to the subclavian or iliac vessels. Certain arteries (e.g., the profunda femoris, profunda brachii, and peroneal arteries) normally do not produce palpable pulses, and API is of limited usefulness in these injuries. Shotgun wounds often are associated with normal APIs despite multiple small arterial wounds; catheter-based angiography is the preferred diagnostic modality in this group. As with formal angiography, API cannot detect venous injuries.
Peripheral Vascular Injury
Perspective
Epidemiology
Principles of Disease
Blunt Trauma
Complete Occlusive Injury
Nonocclusive Injuries
Clinical Features
Hard Findings of Vascular Injury
Soft Findings of Vascular Injury
High-Risk Injuries
Physical Examination
Diagnostic Strategies
Plain Radiography
Pulse Oximetry
Ankle-Brachial Index and Arterial Pressure Index
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree