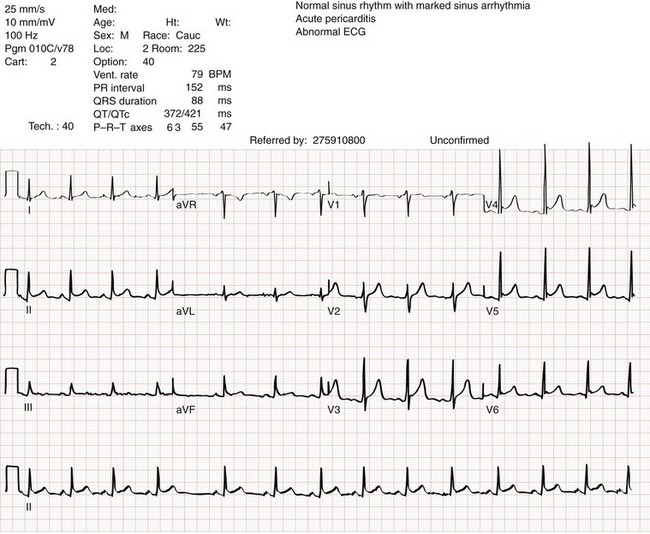
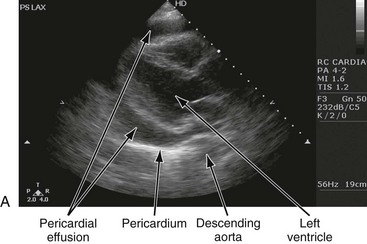
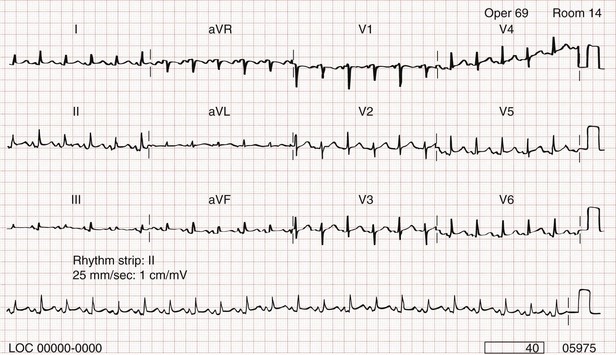
Chapter 82 Our knowledge of pericardial function and disease has increased greatly since Hippocrates described the pericardium in 460 BC as “a smooth tunic that envelops the heart and contains a small amount of fluid resembling urine.” Galen provided the first description of a pericardial effusion and performed the first pericardial resection.1 Lancisi first described the appearance of constrictive pericarditis at autopsy in 1728. Also in the 18th century, Laennec said, “There are few diseases attended by more variable symptoms and more difficult to diagnose than [pericarditis].”1 In 1935, Beck described the clinical presentation of cardiac tamponade, known as Beck‘s triad (hypotension, jugular venous distention, and muffled heart sounds).2 Despite the passage of time and the modern availability of many diagnostic tools, the diagnosis and treatment of pericardial disease still present a challenge. Each of the disorders listed in Box 82-1 can produce acute pericarditis, with or without pericardial effusion. In addition, most of these disorders can progress to cardiac tamponade or constrictive pericarditis. Pericardial Anatomy and Physiology The normal pericardium envelops the heart and attaches to the great vessels. It consists of parietal and visceral layers, with a narrow potential space between them. The visceral layer or epicardium adheres to the myocardium. It is separated from the parietal layer by a potential space. Each layer is 1 or 2 mm thick and is composed of elastic fibers. The position of the heart within the chest is stabilized by the attachment of the parietal pericardium to the sternum, the diaphragm, and the vertebral column. Its blood supply comes from the internal mammary artery, and its nerve supply from the phrenic nerve.3 There is no single test that is diagnostic for pericarditis. The ECG is the most reliable diagnostic tool. It evolves through stages that occur over time. The first stage occurs in the first hours to days of illness. It includes diffuse ST segment elevation seen in leads I, II, III, aVL, aVF, and V2 through V6 and also reciprocal ST segment depression in aVR and V1. Most patients with acute pericarditis have concurrent PR segment depression (Fig. 82-1). In the next stages, the ST and PR segments normalize, but the T waves flatten, and then there is deep, symmetrical T wave inversion. At the last stage, the ECG reverts to normal, although the T wave inversions may become permanent.4 If a specific cause of pericarditis is found, therapy should be specific for that cause. Otherwise, therapy for acute pericarditis is symptomatic. Anti-inflammatory therapy will reduce pain, and a nonsteroidal anti-inflammatory drug (NSAID) is the treatment of first choice. Ibuprofen has the best side effect profile, but other NSAIDs should be equally effective. The patient will often report significant pain relief from the analgesic effect of the ibuprofen while in the ED, even before onset of the anti-inflammatory effect. A dose of 600 mg four times a day for 1 week is a good initial therapy. If the chosen NSAID is not effective within 1 week, a different class of NSAIDs should be tried, such as indomethacin 25 mg three times a day for 1 week. Colchicine (1.2 initially then 0.6 mg daily for up to 6 months) is effective for recurrent pericarditis, and steroid therapy (1 mg/kg daily) has shown mixed results.5,6 The clinical course of pericarditis is variable: 60% of patients have complete recovery within 1 week, and almost 80% have complete recovery within 3 weeks. Patients with fever, pericardial effusion, a subacute course, or failure of initial NSAID treatment have a worse prognosis. Eighteen percent of patients can have recurrent pericarditis that may require serial echocardiography to exclude effusion or tumor.6 Patients with uremic pericarditis have chest pain, unexplained fever, and possibly a coarse friction rub. They also may have significant effusions. The ECG in uremic pericarditis is often normal because little epicardial inflammation occurs.7 In a dialysis patient, cardiac enlargement on chest radiograph in the absence of signs of volume overload or congestive heart failure (CHF) should prompt consideration of pericardial effusion. An echocardiogram will provide the definitive answer. Pericardiocentesis may be needed to exclude infection. Dressler reported a syndrome of fever, pleuritis, leukocytosis, pericardial friction rub, and chest radiograph evidence of new pericardial or pleural effusions in post-MI patients.8 Frequent relapses and a high incidence of friction rubs led Dressler to describe this syndrome as a delayed complication of MI in contrast to the well-known syndrome of early post-MI pericarditis. The cause of late post-MI pericarditis (Dressler’s syndrome) may be immunologic, but the syndrome may also occur with pulmonary embolus and after pericardiotomy. Anticoagulants should be discontinued to reduce the risk of hemorrhage. Delayed post-MI pericarditis is treated with NSAIDs such as ibuprofen 600 mg four times per day or indomethacin 25 mg three times per day. Malignant pericardial tumors typically manifest late, which complicates diagnosis and treatment. Malignant involvement of the pericardium is observed in 3.4% of general autopsies and 2 to 31% of cancer autopsies. The most common causes are lung cancer (30%), breast cancer (23%), lymphoma (17%) and leukemia (9%). Primary malignancies of the pericardium are rare.9 Ten percent of all patients with cancer develop cardiac tamponade.10 Cardiac tamponade should be suspected in patients with penetrating chest wounds. It is also common in patients with uremic pericarditis. Cardiac tamponade is a physiologic continuum reflecting the amount of fluid, the rate of accumulation, and the nature of the heart. The three stages necessary for tamponade to develop are fluid filling the recesses of the parietal pericardium, fluid accumulating faster than the rate of the parietal pericardium’s ability to stretch, and accumulation that exceeds the body’s ability to increase blood volume to support right ventricle filling pressure. The final result is increased pericardial pressure, which causes decreased ventricle compliance and decreased flow of blood into the heart. The reduction of blood inflow into the right ventricle results in decreased stroke volume that leads to decreased cardiac output.10 The most important factor in the development of tamponade is the rate of fluid accumulation. Cardiac tamponade symptoms are often nonspecific, but particularly include chest pain, cough, or dyspnea, any of which may be progressive and severe. The classic triad of cardiac tamponade signs described by Beck is hypotension, distended neck veins, and muffled heart sounds.11 These signs may not be present if tamponade develops quickly. The chest radiograph shows cardiomegaly only if there is a large accumulation of fluid (250 mL). The ECG classically shows decreased voltage or electrical alternans (Fig. 82-3), but the latter is rare. Echocardiography confirms the diagnosis when an effusion and paradoxical systolic wall motion are seen. Thermodilution catheters can also be diagnostic, showing equalization of right and left ventricular pressures. Initial treatment includes volume augmentation to the right ventricle with intravenous fluids to increase the filling pressure to overcome the pericardial constriction. Pericardiocentesis or pericardial window is the treatment of choice. Enough fluid should be withdrawn to stabilize the patient. If tamponade recurs, pericardiocentesis may be repeated, or a drainage catheter may be left in the pericardial space. A pericardiectomy ultimately may be necessary. Cardiac tamponade has a high mortality that depends on the severity and nature of the underlying disease, the time course of onset, and the rapidity of diagnosis and intervention. Traumatic cardiac tamponade is discussed in Chapter 45. Tuberculous pericarditis is estimated to occur in 1 or 2% of patients with pulmonary tuberculosis.12 In Africa, it is the most common cause of pericarditis. In countries in which tuberculosis is not a major health problem, tuberculous pericarditis is most common in patients who are socioeconomically deprived or immunodeficient. In many patients the chest radiograph shows an enlarged cardiac silhouette without a pulmonary infiltrate. Pericardial fluid aspirates reveal acid-fast bacilli by smear or culture (which may require 4-6 weeks to become positive) in approximately 50% of cases. Diagnostic workup should include assessment for human immunodeficiency virus (HIV). Patients with tuberculous pericarditis should be hospitalized and observed for evidence of cardiac tamponade. Triple-drug therapy should be started in the hospital and continued for at least 9 months. Patients with chronic pericardial effusions may benefit from oral prednisone therapy. The mortality rate is approximately 15% in HIV-negative patients and 20 to 35% in HIV-positive patients.12
Pericardial and Myocardial Disease
Pericardial Disease (Pericarditis)
Etiology
Principles of Disease
Idiopathic Pericarditis
Diagnostic Strategies
Management and Disposition
Complications
Uremic Pericardial Disease
Clinical Features and Diagnostic Strategies
Post–Myocardial Infarction Pericarditis
Neoplastic Pericardial Disease
Pericardial Effusion
Cardiac Tamponade
Symptoms and Signs
Diagnostic Strategies
Management and Prognosis
Purulent Pericarditis
Tuberculous Pericarditis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Anesthesia Key
Fastest Anesthesia & Intensive Care & Emergency Medicine Insight Engine