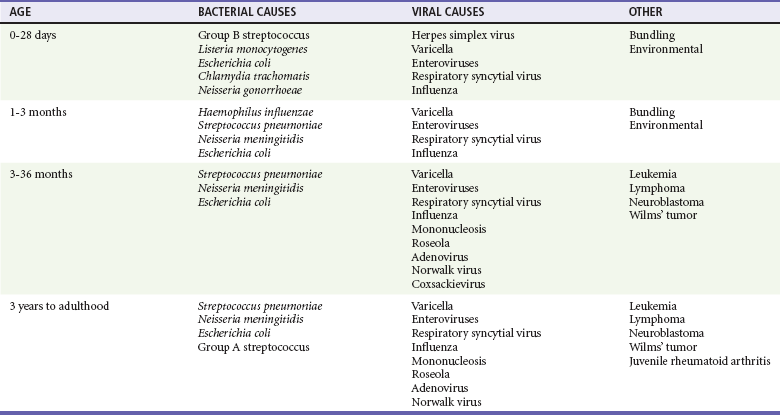
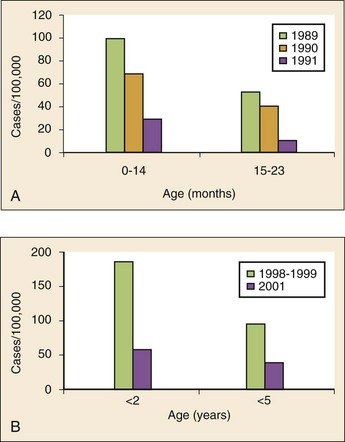
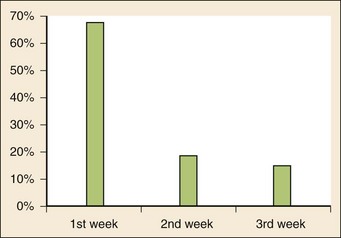
Chapter 167 Fever is the most common chief complaint of pediatric patients presenting to the emergency department (ED), accounting for up to 20% of ED visits. Most cases of fever are viral in origin, are benign in course, and resolve spontaneously. Fever tends to be of a higher clinical importance in younger children as they are immunologically immature and incompletely vaccinated. Management of children presenting to the ED varies dramatically by the age of the child, with the following common, albeit arbitrary, divisions: 0 to 28 days, 1 to 2 months, 2 to 3 months, 3 to 6 months, 6 to 36 months, and 3 years to adulthood.1 These divisions reflect differing immunologic and vaccination milestones as well as the spectrum of age-specific pathogens. The cause of fever varies with the age of the child (Table 167-1). The majority of pediatric fever is due to infections, and most infections are attributable to a viral source. Upper respiratory infections, viral gastroenteritis, croup, bronchiolitis, stomatitis, roseola, infectious mononucleosis, and varicella are all known causes of fever. Most viral illnesses are benign and self-limited, but infection with herpes simplex virus (HSV) or respiratory syncytial virus (RSV), particularly in the first month of life, can lead to significant morbidity and mortality. Bacterial disease is also an important cause of fever in children. Serious bacterial illness (SBI) is typically defined as the presence of pathogenic bacteria in a previously sterile site and includes urinary tract infection (UTI), bacteremia, meningitis, osteomyelitis, bacterial gastroenteritis, bacterial pneumonia, cellulitis, and septic arthritis. Studies have found the risk of SBI in febrile infants younger than 3 months with a temperature above 38.0° C to be between 6 and 10%; children younger than 28 days have the highest incidence.2 Pathogens change during early infancy, with vertical transmission of organisms such as group B streptococcus, Listeria monocytogenes, and HSV being more common in neonates. By 1 to 2 months of age, organisms such as Streptococcus pneumoniae, Neisseria meningitidis, and urinary pathogens (Escherichia coli or Enterococcus) become more common. In all children younger than 3 months, the urinary tract is the most common site of infection, followed by bacteremia and meningitis. UTIs are more common in white girls compared with other races and are of higher prevalence in patients in whom no source for infection is found and who have higher temperatures (i.e., >39.0° C).3 Children younger than 3 months may present with an apparent viral syndrome and still harbor serious bacterial illness. Levine and colleagues studied 1248 infants younger than 60 days who had temperatures above 38.0° C. Of these children, 22% were positive for RSV. Although, overall, children with documented RSV had a lower incidence of concomitant SBI than did those without RSV (12.5 vs. 7%), there was no significant difference in rates of SBI in children younger than 28 days (14.2% in RSV-negative neonates vs. 10.1% in RSV-positive infants). Most of the bacterial infections were UTIs.4 Older children 3 to 36 months of age with recognizable viral syndromes (e.g., croup, bronchiolitis, varicella, stomatitis) have a very low incidence of bacteremia. Greenes and co-workers found that among 1347 patients with temperature above 39.0° C who had a recognizable viral syndrome, the risk of bacteremia was 0.2%.5 Occult bacteremia describes the presence of pathogenic bacteria in the bloodstream of a well-appearing febrile child in the absence of a focus of infection; it was first described as a clinical entity in the 1970s.6 The term typically refers to children 3 to 36 months of age who are highly febrile (>39.0° C) but appear well. Before the adoption of the conjugate vaccines against Haemophilus influenzae type b and S. pneumoniae, the incidence of bacteremia in this population was approximately 5%.7,8 Vaccination has proved remarkably effective, nearly eradicating H. influenzae type b as a significant pathogen and greatly reducing the burden of pneumococcal disease (Fig. 167-1).9–11 Currently, the rate of occult bacteremia is below 1%, with pathogens such as N. meningitidis becoming proportionally more prevalent. Continued surveillance is ongoing to ensure that there is not a rise in invasive disease caused by nonvaccine serotypes. Urinary pathogens, occurring in 2% of febrile children younger than 5 years, continue to be an important source of bacterial illness in infants and children. Rates are highest in boys younger than 6 months (2.7%) and girls younger than 12 months (6-8%).12,13 Bacterial illness in school-age children and adolescents includes focal infections, such as streptococcal pharyngitis, cellulitis, and pneumonia as well as bacteremia and meningitis. N. meningitidis has a bimodal distribution, with the highest incidence in children younger than 12 months (9.2/100,000 population). A second peak occurs during adolescence, when the rate of illness is 1.2/100,000 population, with a significant proportion of cases occurring in college students who reside in a dormitory setting (3.2/100,000 population).14 In dealing with a febrile child, history taking should focus on the length of illness, presence of localizing symptoms, and any pertinent past medical history. In infants younger than 28 days, birth history, particularly the presence of potentially transmittable maternal infection (HSV or group B streptococcus), is critically important. Immunization status, sick contacts, use of antipyretics before evaluation, and prior use of antibiotics are also important historical items. Defervescence after acetaminophen administration has not been shown to reliably exclude bacteremia in children of any age.15 Prior antibiotic use may mask the classic findings in diseases such as meningitis. Cough and congestion may suggest pneumonia or viral upper respiratory infection, whereas a harsh, barking, or seal-like cough is often a predominant complaint in viral laryngotracheitis (croup). Parents may report vomiting and diarrhea as a component of gastroenteritis or the presence of sore throat and lymphadenopathy with viral or streptococcal pharyngitis. Decreased oral intake or decreased urine output is frequently a complaint in gastroenteritis, but it may also be seen in patients with stomatitis as the painful aphthous ulcerations in the mouth make fluid intake difficult. Any history of lethargy, irritability, or altered mental status can be elicited with severe dehydration but raises the specter of meningitis or encephalitis. A rash occurs in many viral illnesses, such as roseola, but it also may be seen in life-threatening conditions, such as meningococcemia, Rocky Mountain spotted fever, and toxic shock syndrome. Complaints of headache and neck pain (meningitis or encephalitis) or ear pain (otitis media) are also important historical points. The physical examination of the febrile child should begin with a complete set of vital signs, including pulse oximetry. Hypoxia or significant respiratory distress manifested by tachypnea, grunting respirations, nasal flaring, or retractions may accompany sepsis or pulmonary infection. Stridor can be seen with croup but also with retropharyngeal abscess, epiglottitis, or bacterial tracheitis. Signs of shock, such as hypotension and poor peripheral perfusion, should be noted. Children typically mount a tachycardic response to fever, and hypotension is often a late and dire finding. Tachycardia is often due to the fever itself, but tachycardia out of proportion to the degree of fever is often seen in other conditions, such as early shock, myopericarditis, and dehydration. Greenes and colleagues found that in infants younger than 12 months, heart rate increases linearly by 9.6 beats/minute with each 1° C increase in body temperature; however, caution must be exercised in attributing tachycardia to fever alone.16 Once oxygenation, ventilation, and perfusion have been assessed and deemed adequate, the physical examination should focus on a thorough search for focal infection. In young infants, particularly those younger than 3 months, and in children who lack immunocompetence, fever may be the only presenting sign of serious illness, including meningitis. The physical examination in this age group is sufficiently insensitive to exclude SBI, and clinicians should not be falsely reassured by a normal physical examination in small children. Numerous laboratory and radiographic studies can be used to evaluate the febrile child. In general, testing should be directed at identification of the source of infection or evaluation for complications. Several guidelines exist for the evaluation of febrile children, although there is marked variation in adherence to these guidelines. Pantell and associates found that office-based practitioners followed published guidelines only 42% of the time in the evaluation of febrile children.17 Clinicians with less experience and those based in the hospital tend to order more tests compared with more experienced clinicians and those practicing in an office setting. An elevated white blood cell (WBC) count (>15,000/mm3) can be an indicator of bacteremia but is also present in many viral illnesses. Leukopenia (<5000/mm3) can also be a sign of SBI or early sepsis. Pneumococcal infection is classically associated with leukocytosis, whereas infection with N. meningitidis and H. influenzae may be present even with normal WBC counts. Lee and colleagues found that the rate of pneumococcal bacteremia increased from 0.5% in highly febrile children (>39.0° C) with a WBC count between 10,000 and 15,000/mm3 to 3.5% if the WBC count was 15,000 to 20,000/mm3 and up to 18% with a WBC count above 30,000/mm3.18 The WBC differential has also been used to risk stratify febrile children in various models; an increase in polymorphonuclear leukocytes and immature band forms increases the risk of bacterial disease. A rise in polymorphonuclear leukocytes is also seen early in some viral infections. An absolute neutrophil count (ANC) above 10,000/mm3 suggests increased risk of pneumococcal bacteremia in febrile children (0.8% for children with an ANC below 10,000/mm3 vs. 8% for children with an ANC above 10,000/mm3).19 A blood culture is a useful diagnostic test when bacteremia is suspected. In infants and children, blood should be obtained after sterile preparation of the skin, which includes an alcohol preparation and swabbing of the skin with povidone-iodine solution, which is then allowed to dry. Many centers obtain blood for culture during intravenous catheter placement after sterile preparation of the skin has been performed. This technique has the advantage of eliminating a second venipuncture solely to obtain blood for culture, although the rates of contamination with this technique have been shown to be higher in children (9.1 vs. 2.8%).20 The risks of contamination should be weighed against the ability to obtain blood through a separate venipuncture. The yield of a single blood culture in infants and small children is actually good. The routine sending of more than one sample is generally not needed, and bacteremia is often accurately detected even if only 0.5 to 1 mL of blood is obtained. The advent of automated blood culture systems led to the identification of true pathogens more quickly than by traditional methods, often within 24 hours. Pathogens isolated in the first 24 hours are more likely to be true pathogens than are bacteria isolated after 24 hours.21 UTI is defined as the combination of bacteriuria and pyuria. Bacteriuria in the absence of WBCs on microscopic examination represents asymptomatic bacteriuria. Urine is typically analyzed with a dipstick, followed by microscopic analysis of a centrifuged specimen of urine. Hoberman and colleagues described the test characteristics of an “enhanced” urinalysis, which is examination with a hemocytometer of an unspun specimen of urine for pyuria (defined as >10 WBCs per high-power field) or the presence of any bacteria per high-power field in Gram’s stain of unspun urine. They reported a negative predictive value of 99.8%, perhaps making urine culture unneeded if pyuria and bacteriuria are absent by use of the enhanced urinalysis method.22 Many centers are not using this enhanced method. Because dipstick and microscopic analysis have lower sensitivities, most experts recommend sending urine for culture in high-risk groups (febrile girls <24 months of age, uncircumcised boys <12 months of age, and circumcised boys <6 months of age). A sample of cerebrospinal fluid (CSF) should be obtained from any child with signs and symptoms of meningitis. Fluid should be obtained with the smallest pencil-point or noncutting spinal needle possible (typically a 22-gauge spinal needle) and sent for cell counts, manual differential, Gram’s staining, culture, and measurement of CSF protein and glucose concentrations. Meningoencephalitis due to HSV is a potential cause of fever, particularly in children, and if it is suspected, sending of CSF for HSV polymerase chain reaction testing is indicated. The CSF in bacterial meningitis typically contains more than 1000 WBCs/mL, although there is considerable overlap in the CSF profile of bacterial and viral meningitis, making a determination of viral or aseptic meningitis difficult on the basis of CSF parameters such as cell count, protein, and glucose; thus CSF culture of a pathogenic bacterium is the “gold standard.” A prediction rule has been developed and validated to differentiate bacterial from aseptic meningitis.23 Children who lack all of the following criteria have a low risk (0.1%) of bacterial meningitis: positive CSF Gram’s stain, CSF ANC of at least 1000 cells/mL, CSF protein concentration of at least 80 mg/dL, peripheral blood ANC of at least 10,000 cells/mL, and history of seizure before or at the time of presentation. This may obviate the need for empirical antibiotic therapy and hospital admission in some children who are at low risk for bacterial meningitis. Contraindications to lumbar puncture include cellulitis over the proposed site of puncture, cardiopulmonary instability, bleeding diathesis or platelet count below 50,000/µL, focal neurologic deficits, and signs of increased intracranial pressure, including papilledema. In these patients, lumbar puncture should be deferred until the child is stable, and blood should be obtained for culture while the child is treated empirically, recognizing that up to 50% of children with meningitis will not have bacteremia.24 CSF contaminated by blood, or a traumatic lumbar puncture, can make interpretation of cell counts and differentials difficult. In these cases, fluid should be obtained for Gram’s stain and culture and the child treated presumptively for meningitis until culture data are available. Nigrovic and co-workers have shown that risk factors for a traumatic lumbar puncture include operator experience, excessive patient movement during the procedure, advancement of the needle with the stylet in place, and lack of local anesthesia.25 Chest radiographs may be useful in the workup of the febrile child and are indicated when hypoxemia, respiratory distress, tachypnea, or focal findings on lung examination are present. Children younger than 6 months may present with tachypnea as the sole finding of bacterial pneumonia. Truly occult pneumonia can also occur, particularly in the highly febrile child (>39.0° C) without apparent source of fever. Bachur and associates found that 26% of children with temperature above 39.0° C and WBC counts higher than 20,000 had radiographic evidence of pneumonia.26 Of note, this study was done in the era before the conjugate pneumococcal vaccine. Many clinical laboratories have the ability to perform rapid viral antigen testing for such common pediatric viral illnesses as influenza A and B and RSV. The presence of a viral “source” for the fever in an ill child may obviate the need for expensive, painful, and lengthy diagnostic workups for bacterial processes. Bonner and colleagues evaluated the impact of physician knowledge of the presence of a positive rapid influenza assay result.27 The investigators studied 391 patients aged 2 months to 21 years who presented with classic signs and symptoms of influenza. Of these patients, more than half had positive rapid assays for influenza, and the investigators noted that significantly fewer laboratory and radiographic studies were ordered if the treating clinician was aware of these results. There were also fewer antibiotics prescribed. A large multicenter trial of febrile infants 60 days of age or younger revealed a decreased risk (2.5 vs. 11.7%) if the infant was influenza positive.28 General Approach to the Febrile Infant and Child Every effort should be made to obtain appropriate specimens for culture (blood and urine), even in the critically ill child, before antibiotic administration. Lumbar puncture may be deferred in the critically ill child until stabilization occurs. Empirical antibiotic therapy should be directed at the most likely causative organisms based on age. Sterilization of the CSF occurs quickly once antibiotic administration has been initiated: within 15 minutes to 2 hours in patients with meningococcal meningitis and within 4 to 10 hours in patients with pneumococcal meningitis.29 Children presenting with temperature above 38.0° C who are younger than 28 days are at particularly high risk for bacterial illness, with rates as high as 12% quoted in the literature.30,31 Often, fever is the only manifestation of potentially life-threatening disease, and other signs and symptoms may be exceedingly subtle. This has led to an aggressive approach to diagnostic testing, empirical antibiotic therapy, and hospitalization in this age group, even if the child appears well. Children in this age group often present with nonspecific complaints, such as irritability, lethargy, poor feeding, and grunting. Besides fever, other signs of serious illness include a bulging fontanel, mottled extremities, petechiae, and tachypnea. Bacterial pathogens in this age group include group B streptococcus, L. monocytogenes, N. meningitidis, S. pneumoniae, and E. coli. Viral pathogens, including RSV and HSV, are also important considerations. Neonatal HSV infection in particular carries a high degree of morbidity and mortality and should be considered in any febrile neonate with a maternal history of genital herpes, who appears ill, who presents with fever and seizure, who has cutaneous vesicles on physical examination, or who has evidence of transaminitis or coagulopathy. HSV meningoencephalitis should also be considered in patients with fever and CSF pleocytosis but a negative CSF Gram’s stain. The risk period for HSV disease tends to be between 2 and 12 days of age (Fig. 167-2). Other noninfectious causes of a septic-appearing neonate include the acute salt-wasting crisis associated with congenital adrenal hyperplasia and undiagnosed duct-dependent congenital heart disease.
Pediatric Fever
Perspective
Distinguishing Principles of Disease
History
Physical Examination
Ancillary Testing
White Blood Cell Count
Blood Culture
Urinalysis and Urine Culture
Lumbar Puncture
Chest Radiography
Rapid Viral Antigen Testing
Specific Disorders
Serious Bacterial Illness
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pediatric Fever
Only gold members can continue reading. Log In or Register to continue