Anatomic and Physiological Development: Respiratory System
Pulmonary function: Neonates and infants ventilate less efficiently because of more horizontal, pliable ribs and protuberant abdomens. Fewer, smaller airways and alveoli result in reduced lung compliance, increased airway resistance, and increased work of breathing. A cartilaginous ribcage increases chest wall compliance, promoting collapse during inspiration and low residual lung volumes at expiration, limiting O2 reserve during apneic periods and predisposing neonates and infants to atelectasis and hypoxemia. Neonates and infants do not have well-developed responses to hypoxia and hypercapnia; both states may actually depress respiration. Respiratory rate is increased in neonates and falls to adult values by adolescence. Tidal volume and dead space per kilogram remain nearly constant during development.
Airway anatomy: Neonates and infants have proportionately larger heads and tongues, narrower nasal passages, anterior and cephalad larynxes (glottis is at a C4 vertebral level vs. C6 in adults), longer epiglottises, and shorter tracheas and necks. The cricoid cartilage is the narrowest point of the airway in children younger than 5 years of age; in adults, the narrowest point is the glottis.
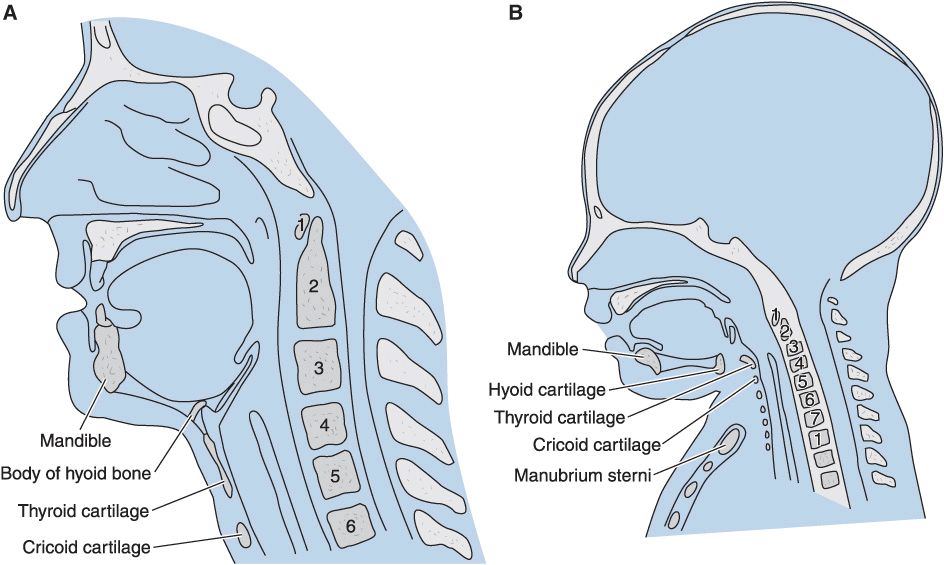
(Reproduced, with permission, from Snell RS, Katz J: Clinical Anatomy for Anesthesiologists. Appleton & Lange, 1988.)
Anatomic and Physiological Development: Cardiovascular System
Stroke volume is relatively fixed by a noncompliant, immature left ventricle in neonates and infants; thus, cardiac output is very sensitive to changes in heart rate. Although basal heart rate is greater than in adults, activation of the parasympathetic nervous system, anesthetic overdose, or hypoxia can quickly trigger bradycardia and profound reductions in cardiac output. Sick infants undergoing emergency or prolonged surgical procedures are particularly prone to episodes of bradycardia that can lead to hypotension, asystole, and intraoperative death. The sympathetic nervous system and baroreceptor reflexes are immature, and the infant cardiovascular system displays a blunted response to exogenous catecholamines. The immature heart is more sensitive to depression by volatile anesthetics and to opioid-induced bradycardia. The vascular tree is less able to respond to hypovolemia with compensatory vasoconstriction. Intravascular volume depletion in neonates and infants may manifest as hypotension without tachycardia.
Age-Related Changes in Vital Signs
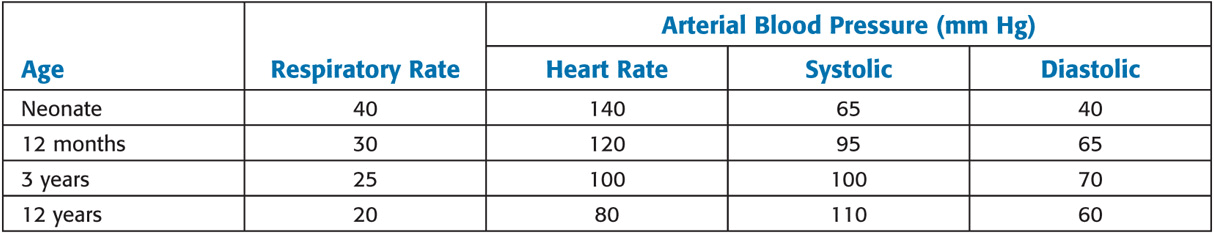
Values are mean averages derived from numerous sources. Normal ranges may include measurements that deviate from these as much as 25% to 50%.
Anatomic and Physiological Development: Renal and Gastrointestinal Systems
Renal function: Kidney function usually approaches normal values (corrected for size) by 6 months to as late as 2 years of age. Premature neonates often have multiple forms of renal deficiency, including decreased creatinine clearance; impaired sodium retention, impaired glucose excretion, and impaired bicarbonate reabsorption; and reduced diluting and concentrating ability. These abnormalities underscore the importance of appropriate fluid administration in the early days of life.
Gastrointestinal (GI) function: Neonates have a relatively increased incidence of gastroesophageal reflux. The immature liver conjugates drugs and other molecules less readily.
Anatomic and Physiological Development: Glucose Homeostasis
Neonates have relatively reduced glycogen stores that predispose them to hypoglycemia. Impaired renal glucose excretion may partially offset this tendency. The neonates at greatest risk for hypoglycemia are premature or small for gestational age, receiving hyperalimentation, or born to mothers with diabetes.
Pharmacologic Differences
Pediatric drug dosing is typically adjusted on a per-kilogram basis. In early childhood, a patient’s weight can be approximated based on age: 50th percentile weight (kg) = (Age × 2) + 9
Weight-adjustment dosing does not factor in the disproportionately larger pediatric fluid compartments, immature hepatic enzyme systems, decreased glomerular filtration rate and renal tubular function, increased organ blood flow, decreased protein for drug binding or higher metabolic rate.
Neonates and infants have greater total water content (70%–75%) than adults (50%–60%). Thus, the volume of distribution for most intravenous (IV) drugs is disproportionately greater in neonates, infants, and young children, and the optimal dose (per kilogram) is usually greater than in older children and adults.
Decreased muscle mass in neonates prolongs some drugs’ durations of action by delaying redistribution to muscle (e.g., fentanyl, thiopental).
Pharmacologic Differences: Inhalational Anesthetics
Faster inhalation induction: Neonates, infants, and young children have relatively greater alveolar ventilation and reduced FRC compared with adults. This greater minute ventilation-to-FRC ratio with greater blood flow to vessel-rich organs contributes to a rapid increase in alveolar anesthetic concentration and speeds inhalation induction. The blood/gas coefficients of volatile agents are lower in neonates than in adults, contributing to faster induction times and potentially increasing the risk of accidental overdose.
Minimum alveolar concentration (MAC): MAC for halogenated agents is greater in infants than in neonates and adults. Nitrous oxide does not appear to reduce MAC of desflurane or sevoflurane in children to the same extent as it does for other agents.
Blood pressure (BP) sensitivity: BP in neonates and infants is more sensitive to volatile agents. This clinical observation has been attributed to immature compensatory mechanisms (e.g., vasoconstriction, tachycardia) and greater sensitivity of the neonatal and infant myocardium to myocardial depressants.
Airway irritation: Halothane and sevoflurane are less likely to irritate the airway or cause breath holding or laryngospasm during induction than other volatile agents.
Respiratory depression: In general, volatile anesthetics appear to depress ventilation more in infants than in older children. Sevoflurane appears to produce the least respiratory depression.
Emergence delirium: Emergence is fastest after use of desflurane or sevoflurane, but both agents are associated with a greater incidence of agitation or delirium upon emergence, particularly in young children. Because of the latter, some clinicians switch to isoflurane for maintenance of anesthesia after a sevoflurane induction.
Sevoflurane: There are no reported instances of renal toxicity attributed to inorganic fluoride production during sevoflurane anesthesia in children. Overall, sevoflurane appears to have a greater therapeutic index than halothane and has become the preferred agent for inhaled induction in pediatric anesthesia.
Pharmacologic Differences: Nonvolatile Anesthetics
Propofol: After weight-adjustment of dosing, infants and young children require larger doses of propofol because of a larger volume of distribution compared with adults. Children have a shorter elimination half-life and higher plasma clearance for propofol. Although recovery from a single bolus is not noticeably different from that in adults, recovery after a continuous infusion may be more rapid. Children may require increased weight-adjusted rates of infusion for maintenance of anesthesia. Propofol is not recommended for prolonged sedation of critically ill pediatric patients because of an association with greater mortality than other agents. “Propofol infusion syndrome” appears more common in children than adults. Its features include rhabdomyolysis, metabolic acidosis, hemodynamic instability, hepatomegaly, and multiorgan failure.
Opioids: Opioids appear to be more potent in neonates than in older children and adults. Unproven possible explanations include “easier entry” across the blood–brain barrier, decreased metabolic capability, or increased sensitivity of the respiratory centers. Morphine sulfate, particularly in repeated doses, should be used with caution in neonates because hepatic conjugation is reduced and renal clearance of morphine metabolites is decreased. Older pediatric patients have relatively greater rates of biotransformation and elimination as a result of high hepatic blood flow. Sufentanil, alfentanil, remifentanil and (possibly) fentanyl clearances may be greater in children than in adults.
Ketamine: Neonates and infants may be more resistant to the hypnotic effects of ketamine, requiring slightly higher doses than adults (but the “differences” are within the range of error in studies); pharmacokinetic values do not significantly differ from those of adults.
Midazolam: Midazolam has the fastest clearance of all the benzodiazepines; however, midazolam clearance is significantly reduced in neonates compared with older children. The combination of midazolam and fentanyl can cause hypotension in patients of all ages.
Pharmacologic Differences: Muscle Relaxants
Faster onset: All muscle relaxants generally have a faster onset (up to 50% less delay) in pediatric patients because of shorter circulation times than adults. In both children and adults, IV succinylcholine (1–1.5 mg/kg) has the fastest onset.
Larger dose requirement: Infants require significantly larger doses of succinylcholine (2–3 mg/kg) because of the relatively larger volume of distribution. With the exclusion of succinylcholine and possibly cisatracurium, infants require significantly smaller muscle relaxant doses than older children. As with adults, a more rapid intubation can be achieved with a muscle relaxant dose that is twice the ED95 dose.
Variable response to nondepolarizing muscle relaxants: The response of neonates to nondepolarizing muscle relaxants is variable. The explanations for this include “immaturity of the neuromuscular junction (in premature neonates) tending to increase sensitivity” (unproven) and a disproportionately larger extracellular compartment reducing drug concentrations (proven). The relative immaturity of neonatal hepatic function prolongs the duration of action for drugs that depend primarily on hepatic metabolism (e.g., pancuronium, vecuronium, and rocuronium).
Approximate ED95 for Muscle Relaxants in Infants and Children During N2O/O2 Anesthesia
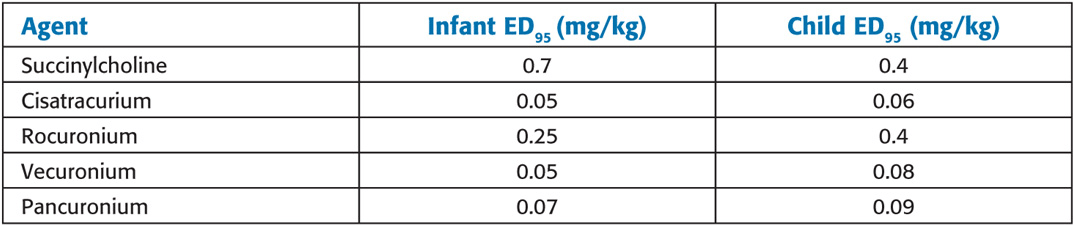
Succinylcholine: Children are more susceptible than adults to cardiac arrhythmias, hyperkalemia, rhabdomyolysis, myoglobinemia, masseter spasm, and malignant hyperthermia associated with succinylcholine. When a child experiences cardiac arrest after succinylcholine administration, immediate treatment for hyperkalemia should be instituted. Prolonged, heroic (e.g., cardiopulmonary bypass) resuscitative efforts may be required. For this reason, succinylcho-line is avoided for routine, elective paralysis for intubation in children and adolescents. Unlike adults, children may have profound bradycardia and sinus node arrest after the first dose of succinylcholine without atropine pretreatment. Atropine (0.1 mg minimum) must always be administered before succinylcholine in children. Generally accepted indications for IV succinylcholine in children include rapid-sequence induction with a full stomach and laryngospasm that does not respond to positive-pressure ventilation (PPV). When rapid muscle relaxation is required before IV access (e.g., with inhaled inductions in patients with “full” stomachs), intramuscular (IM) succinylcholine (4–6 mg/kg) can be used. Atropine IM (0.02 mg/kg) should be administered with IM succinylcholine to reduce the likelihood of bradycardia. Some clinicians advocate midline intralingual succinylcholine (2 mg/kg) as an emergency route.
Rocuronium: Many clinicians consider rocuronium (0.6 mg/kg IV) to be the drug of choice for routine intubation in pediatric patients with IV access because it has the fastest onset of nondepolarizing neuromuscular blocking agents (NMBAs). Larger doses of rocuronium (0.9–1.2 mg/kg) may be used for rapid-sequence induction, but a prolonged duration (up to 90 min) will likely follow. Rocuronium is the only NMBA that has been adequately studied for IM administration (1.0–1.5 mg/kg), but this approach requires 3 to 4 min for onset.
Atracurium and cisatracurium: Atracurium and cisatracurium may be preferred in young infants, particularly for short procedures, because these drugs consistently display short to intermediate durations.
Pediatric Anesthetic Risk
Risk of cardiac arrest: Analysis of the Pediatric Perioperative Cardiac Arrest Registry found the risk of cardiac arrest in pediatric anesthetic cases is approximately 1.4 in 10,000; an overall mortality rate of 26% was reported after cardiac arrest. Approximately 6% children had permanent injury, but the majority (68%) had either no or an only temporary injury. The mortality rate was 4% in American Society of Anesthesiologists physical status (ASA PS) 1 and 2 patients compared with 37% in ASA PS 3 to 5 patients. It is important to note that 33% of patients who had a cardiac arrest were ASA PS 1 to 2. Infants accounted for 55% of all anesthesia-related arrests with those younger than 1 month (i.e., neonates) having the greatest risk. As with adults, two major predictors of mortality were ASA PS 3 to 5 and emergency surgery.
Timing and mechanisms of cardiac arrest: Most (82%) arrests occurred during induction of anesthesia; bradycardia, hypotension, and a low SpO2 frequently preceded arrest. The most common mechanism of cardiac arrest was judged to be medication related. Cardiovascular depression from halothane, alone or in combination with other drugs, was believed to be responsible in 66% of all medication-related arrests. Another 9% was attributable to intravascular injection of a local anesthetic, most often after a negative aspiration test result during attempted caudal injection.
Cardiovascular mechanisms: Presumed cardiovascular mechanisms most often had no clear etiology; in more than 50% of those cases, the patient had congenital heart disease. When a cardiovascular mechanism could be identified, it was most often related to hemorrhage, transfusion, or inappropriate fluid therapy.
Respiratory mechanisms: Respiratory mechanisms included laryngospasm, airway obstruction, and difficult intubation (in decreasing order). Most cases of laryngospasm occurred during induction. Most patients who had airway obstruction or were difficult to intubate had other significant underlying disease.
Equipment-related mechanisms: The most common equipment-related mechanisms that led to a cardiac arrest were complications related to attempted central venous catheterization (e.g., pneumothorax, hemothorax, or cardiac tamponade).
Preoperative Considerations
Preoperative interview: Children present with varying degrees of fear. Rapport may be hard to establish; premedication often helps. Some centers allow someone the child trusts to be present for induction.
Recent upper respiratory tract infection (URI): Children frequently present with evidence of a URI. An infectious cause should be differentiated from an allergic or vasomotor cause. A viral infection 2 to 4 weeks before general anesthesia and endotracheal intubation appears to place the child at an increased risk for perioperative pulmonary complications, such as wheezing, laryngospasm, hypoxemia, and atelectasis. This is more likely if the child has a severe cough, high fever, or a family history of reactive airway disease.
Laboratory tests: Few, if any, preoperative laboratory studies are cost effective. Some pediatric centers require no preoperative tests in healthy children undergoing minor procedures. Most asymptomatic patients with cardiac murmurs do not have significant cardiac pathology. Innocent murmurs may occur in more than 30% of normal children. Consultation with a pediatric cardiologist should be obtained if the patient is symptomatic (e.g., poor feeding, failure to thrive, or easy fatigability); the murmur is harsh, loud, diastolic, holosystolic or radiates widely, or pulses are either bounding or markedly diminished.
Preoperative fasting: Pediatric patients are more prone to dehydration. The incidence of aspiration is reported to be approximately 1 in 1000. There is no convincing evidence that prolonged fasting decreases this risk. Infants are fed breast milk up to 4 h before induction, whereas formula or liquids and a ‘light’ meal may be given up to 6–8 h before induction. Clear fluids are allowed until 2–3 hr before induction. These recommendations are for healthy patients without risk factors for aspiration or decreased gastric emptying.
Premedication: There is great variation in the recommendations for premedication of pediatric patients. Sedative premedication is generally omitted for neonates and sick infants. Children who appear likely to exhibit uncontrollable separation anxiety should be given a sedative, such as midazolam (0.3–0.5 mg/kg, 15 mg maximum). The oral route is generally preferred. Atropine is often administered orally (0.05 mg/kg), IM, or occasionally rectally to decrease the likelihood of brady-cardia during induction. Many anesthesiologists prefer to administer atropine IV at or shortly after induction.
Monitoring
Monitoring requirements for infants and children are generally similar to adults with some minor modifications. Alarm limits should be adjusted appropriately. Smaller electrocardiographic (ECG) electrodes may be necessary so they do not encroach on sterile surgical areas. Blood pressure cuffs must be properly fitted. Precordial stethoscopes provide an inexpensive means of monitoring heart rate, heart sounds, and airway patency.
Pulse oximetry and capnography: Hypoxia from inadequate ventilation remains a common cause of perioperative morbidity and mortality. In neonates, the pulse oximeter probe should preferably be placed on the right hand or earlobe to measure preductal oxygen saturation. Flow-through analyzers are usually less accurate in patients weighing less than 10 kg. Even with aspiration (side-stream) capnographs, the inspired (baseline) CO2 can appear falsely elevated, and the expired (peak) CO2 can be falsely low.
Temperature must be closely monitored in pediatric patients because of the greater risk for malignant hyperthermia and potential for both iatrogenic hypothermia and hyperthermia. The risk of hypothermia can be reduced by maintaining a warm operating room (OR) environment (26°C or higher), warming and humidifying inspired gases, using a warming blanket and warming lights, and warming all IV fluids.
Invasive monitoring: Arterial cannulation and central venous catheterization demand caution. Air bubbles must be removed from pressure tubing, and small volume flushes should be used to prevent air embolism, unintended heparinization, and fluid overload. The right radial artery is often chosen for cannulation in neonates because its preductal location mirrors the oxygen content of the carotid and retinal arteries. A femoral artery catheter may be an alternative in very small neonates and left radial or right or left dorsalis pedis arteries are alternatives in infants. Critically ill neonates may retain an umbilical artery catheter. Urinary output is an important indicator of the adequacy of intravascular volume and cardiac output.
Blood glucose monitoring: Premature or small-for-gestational age neonates and neonates receiving hyperalimentation or whose mothers have diabetes are prone to hypoglycemia and should have frequent blood glucose measurements.
Induction
General anesthesia is usually induced by an intravenous (IV) or inhalational technique. IM ketamine (5–10 mg/kg) induction is reserved for specific situations, such as those involving combative (particularly mentally challenged) children and adults. IV induction is preferred when the patient comes to the OR with a functional IV catheter or will allow awake venous cannulation. Awake or sedated-awake intubation with topical anesthesia should be considered for emergency procedures in neonates and small infants when they are critically ill or a potential difficult airway is present.
Intravenous Induction
The same induction sequence can be used as in adults: propofol followed by a nondepolarizing muscle relaxant or succinylcholine. We recommend that atropine be given routinely before succinylcholine. The advantages of an IV technique include familiarity with the agents, availability of IV access if emergency drugs need to be administered, and rapidity of induction in children at risk for aspiration. Alternatively, intubation can be accomplished with the combination of propofol, lidocaine, and an opiate with or without an inhaled agent, avoiding the need for a muscle relaxant. Muscle relaxants are not needed for placement of laryngeal mask airways (LMAs).
Inhalational Induction
Many children arrive in the OR without an IV line, and nearly all dread needles. The child can usually be coaxed (especially after oral midazolam pretreatment) into breathing an odorless mixture of N2O (70%) and O2 (30%). Sevoflurane or halothane can be added to the anesthetic gas mixture in 0.5% increments. After an adequate depth of anesthesia has been achieved, an IV line can be started and a muscle relaxant (or propofol and opioid) administered to facilitate intubation. Patients typically pass through an excitement stage during which any stimulation can induce laryngospasm. Steady application of 10 cm of positive end-expiratory pressure will usually overcome laryngospasm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





