Patient Positioning and Potential Injuries
Mary E. Warner
Key Points
Related Matter
Brachial Plexus Injury
Upper Extremity Neuropathy
Ulnar Nerve Compression
Ventilation and Perfusion in the Lateral Position
Prone Position Facial
Prone Position
It is very important for clinicians to understand the physiologic and potential pathologic consequences of patient positioning. A number of studies of large surgical populations have provided information on the frequency and natural history of rare
perioperative events such as neuropathies and vision loss. However, these studies frequently have provided insufficient data to allow speculation as to potential mechanisms of injury. Based on the findings of these studies, investigators are seeking to confirm mechanisms of injury and the efficacy of novel interventions to decrease the frequency of these perioperative events. Until these investigations are complete, the etiologic mechanisms for many potential positioning-related complications remain unknown.
perioperative events such as neuropathies and vision loss. However, these studies frequently have provided insufficient data to allow speculation as to potential mechanisms of injury. Based on the findings of these studies, investigators are seeking to confirm mechanisms of injury and the efficacy of novel interventions to decrease the frequency of these perioperative events. Until these investigations are complete, the etiologic mechanisms for many potential positioning-related complications remain unknown.
The lack of solid scientific information on basic mechanisms of positioning-related complications often leads to medicolegal entanglements. Notations on anesthesia and operating room records may be absent or uninformative. Careful descriptive notations about positions used during anesthesia and surgery, as well as brief comments about special protective measures such as eye care and pressure-point padding, are useful to include on the anesthesia record. In potentially complicated or contentious circumstances, a separate brief description of care documented in the patient’s record is advisable. Only in this manner can subsequent inquiries be properly answered on behalf of either the patient or the anesthesiologist. When credible, expanded knowledge that further delineates mechanisms of positioning-related complications is available, these issues and the care of patients will be improved.
General Principles
Viruses have been associated with central as well as peripheral neuropathies that develop in the perioperative period. As noted above, immunosuppression is present in a fairly significant proportion of patients undergoing major surgical procedures. Anesthetics, blood products, and even antibiotics have been shown to result in varying degrees of immunosuppression.3 This immunosuppression may provide opportunities for existing viruses or newly introduced viruses to activate, particularly in neural tissues. For example, the onset of shingles may be more frequent in surgical compared to general populations.4
Positioning can, of course, cause tissue damage. Stretch of neural tissue may be an important factor in the development of peripheral and central neuropathies. Stretch of many mammalian nerves to 5% greater than their normal resting length has been shown repeatedly to lead to ischemia by reducing both arteriole and venule blood flow. The kinking of the arterioles and venules associated with neuronal stretch leads to ischemia. If the ischemia is prolonged, it may result in permanent neural damage. The impact of stretch on other soft tissues is less well documented and would be highly dependent on the type of tissue and amount of stretch.
Point pressure on any soft tissue may reduce local blood flow and cause ischemia. There are many ways to reduce point pressure, but the most commonly used involve padding. While there may be distinct differences in mechanical properties of various padding materials (e.g., gels, foam, textiles, and others), none have been proven to be significantly better than the others in reducing the frequency or severity of nerve or soft tissue damage perioperatively. The basic principle is to use any of these materials to protect nerves and soft tissues from point pressure.
Supine Positions
Variations of Supine Positions
Supine
Horizontal
In the traditional supine position, the patient lies on his or her back with a small pillow beneath the head (Fig. 28-1). The arms are either comfortably padded and restrained alongside the trunk or abducted on well-padded arm boards. Either arm (or both) may be extended ventrally and the flexed forearm secured to an elevated frame in such a way that perfusion of the hand is not compromised, no skin-to-metal contact exists to cause electrical burns if a cautery is used, and the brachial neurovascular bundle is neither stretched nor compressed at the axilla. The lumbar spine may need padded support to prevent a postoperative backache (see “Complications of Supine Positions”). Bony contact points at the occiput, elbows, and heels should be padded. Fortunately, most modern surgical tables have mattress pads that are sufficiently buoyant and thick to allow dispersion of point pressure.
Although the horizontal supine posture has a long history of widespread use, it does not place hip and knee joints in neutral positions and is poorly tolerated for prolonged periods by an immobilized, awake patient.
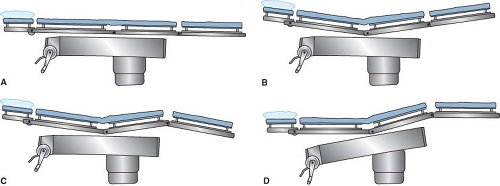
Contoured
A contoured supine posture (Fig. 28-2) has been termed the lawn chair position. It is established by arranging the surface of the operating table so that the trunk–thigh hinge is angulated approximately 15 degrees and the thigh–knee hinge is angulated a similar amount in the opposite direction. Alternatively, a rolled towel, pillow, or blanket can be placed beneath the patient’s knees to keep them flexed. The patient of average height then lies comfortably with hips and knees flexed gently.
Lateral Uterine or Abdominal Mass Displacement
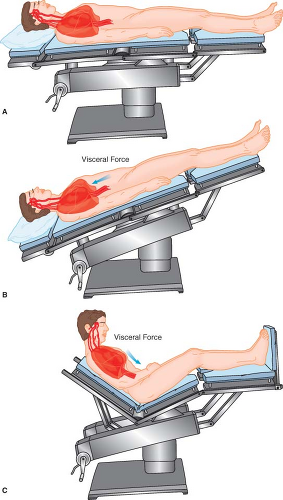
With a patient in the supine position, a mobile abdominal mass, such as a very large tumor or a pregnant uterus, can rest on the great vessels of the abdomen and compromise circulation. This is known as the aortocaval syndrome or the supine hypotensive
syndrome. A significant degree of perfusion can be restored if the compressive mass is rolled toward the left hemiabdomen by leftward tilt of the tabletop or by a wedge under the right hip.
syndrome. A significant degree of perfusion can be restored if the compressive mass is rolled toward the left hemiabdomen by leftward tilt of the tabletop or by a wedge under the right hip.
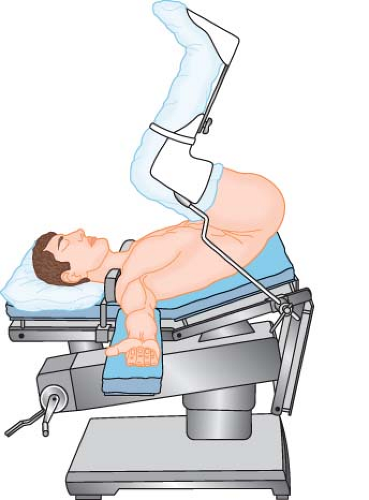
Lithotomy
Standard
In the standard lithotomy position (Fig. 28-3), the patient lies supine, typically with one or both arms extended laterally to <90 degrees on arm boards. Each lower extremity is flexed at the hip and knee, and both limbs are simultaneously elevated and separated so that the perineum becomes accessible to the surgeon. For many gynecologic and urologic procedures, the patient’s thighs are flexed approximately 90 degrees on the trunk and the knees are bent sufficiently to maintain the lower legs nearly parallel to the floor. More acute flexion of the knees or hips can threaten to angulate and compress major vessels at either joint. In addition, hip flexion to >90 degrees on the trunk has been shown to increase stretch of the inguinal ligaments.5 Branches of the lateral
femoral cutaneous nerves often pass directly through these ligaments and can be impinged and become ischemic within the stretched ligament.
femoral cutaneous nerves often pass directly through these ligaments and can be impinged and become ischemic within the stretched ligament.
Numerous devices are available to hold legs that are elevated during obstetric delivery or perineal operations. Each device should be fitted to the stature of the individual patient. Care should be taken to ensure that angulations or edges of the padded holder do not compress the popliteal space or the upper dorsal thigh. Compartment syndromes of one or both lower extremities have resulted from prolonged use of the lithotomy position with various types of support devices.
When the legs are to be lowered to the original supine position at the end of the procedure, they should first be brought together at the knees and ankles in the sagittal plane and then lowered slowly together to the tabletop. This minimizes torsion stress on the lumbar spine that would occur if each leg were lowered independently. It also permits gradual accommodation to the increase in circulatory capacitance, thereby avoiding sudden hypotension.
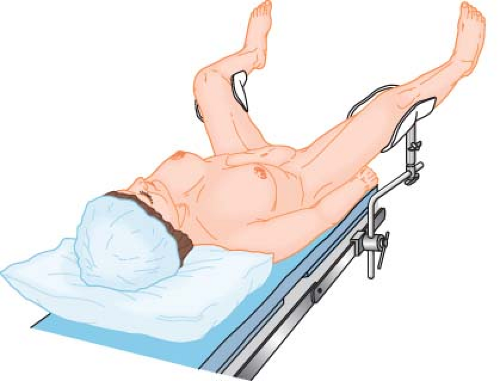 Figure 28.4. Low lithotomy position for perineal access, transurethral instrumentation, or combined abdominoperineal procedures. |
Low
For most urologic procedures and for many procedures that require simultaneous access to the abdomen and perineum, the degree of thigh elevation in the lithotomy position is only approximately 30 to 45 degrees (Fig. 28-4). This reduces perfusion gradients to and from the lower extremities and improves access to a perineal surgical site for members of the operating team who may need to stand at the lateral aspect of either leg.
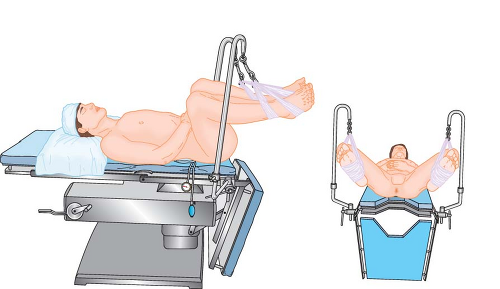
High
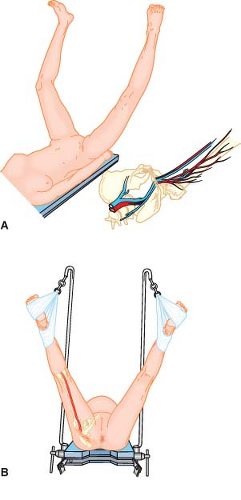
Some surgeons prefer to improve access to the perineum by suspending the patient’s feet from high poles. The effect is to have the patient’s legs almost fully extended on the thighs (Fig. 28-5) and the thighs flexed 90 degrees or more on the trunk. The posture produces a significant uphill gradient for arterial perfusion into the feet, requiring careful avoidance of systemic hypotension. There is considerable variation in lower extremity perfusion pressure in volunteers placed in high lithotomy positions; however, they all tend to have low perfusion pressures.6,7 Less mobile patients may tolerate this posture poorly because of angulation and compression of the contents of the femoral canal by the inguinal ligament (Fig. 28-5A), or stretch of the sciatic nerve (Fig. 28-5B), or both.
Exaggerated
Transperineal access to the retropubic area requires that the patient’s pelvis be flexed ventrally on the spine, the thighs almost forcibly flexed on the trunk, and the lower legs aimed skyward so they are out of the way (Fig. 28-6). The result places the long axis of the symphysis pubis almost parallel to the floor. This exaggerated
lithotomy position stresses the lumbar spine, produces a significant uphill gradient for perfusion of the feet, and may restrict ventilation because of abdominal compression by bulky thighs. It can be tolerated under anesthesia but rarely can be assumed by an awake patient. Control of ventilation is usually necessary. If pre-existing painful lumbar spine disease is present, an alternative surgical position may need to be chosen beforehand to avoid severely accentuating the lumbar distress after surgery. This position has been associated with a very high frequency of lower extremity compartment syndrome.8 Maintenance of adequate perfusion pressure in the legs is important.
lithotomy position stresses the lumbar spine, produces a significant uphill gradient for perfusion of the feet, and may restrict ventilation because of abdominal compression by bulky thighs. It can be tolerated under anesthesia but rarely can be assumed by an awake patient. Control of ventilation is usually necessary. If pre-existing painful lumbar spine disease is present, an alternative surgical position may need to be chosen beforehand to avoid severely accentuating the lumbar distress after surgery. This position has been associated with a very high frequency of lower extremity compartment syndrome.8 Maintenance of adequate perfusion pressure in the legs is important.
Complications of Supine Positions
Pressure Alopecia
Prolonged compression of hair follicles can produce hair loss. Abel and Lewis 9 described patients who had pain, swelling, and exudation where the occiput had been supporting the weight of the head for long periods in a head-down position. Alopecia occurred between the 3rd and 28th postoperative day; regrowth was complete within 3 months. Use of tight head straps to hold anesthetic face masks and prolonged hypotension and hypothermia have also been associated with compression alopecia.10 Frequently turning the patient’s head during long operations11 and use of padded, soft head supports are recommended to reduce the risks of this complication.
Pressure-point Issues
Weight-bearing bony prominences can produce ischemic necrosis of overlying tissue unless proper padding is applied. Hypothermia and vasoconstrictive hypotension may enhance the process. The heels, the elbows, and the sacrum are particularly vulnerable. The use of a variety of pads (e.g., foam or gel) may disperse point pressure if used for protection. Although their use may protect against skin and soft tissue compression and ischemia, there are no studies that have proven their use to be beneficial in reducing peripheral neuropathies in the perioperative period.
Brachial Plexus and Upper Extremity Neuropathies
Brachial Plexus Neuropathy
Root Injuries
Sternal Retraction
Frequently, the patient undergoing a median sternotomy has both arms padded and secured alongside the torso. An alternative is to have both arms abducted.12 Vander Salm et al.13,14 described first rib fractures and brachial plexus injuries associated with median sternotomies. They related the extent of the injury to the amount of retractor displacement of the rib, with the most severe injury being caused by displacement sufficient to produce a first rib fracture. Roy et al.,15 in a study of 200 consecutive adults scheduled for cardiac surgery via a median sternotomy, positioned the left arm either abducted and padded on an arm board with the palm supinated or secured by a draw sheet alongside the trunk; the right arm was always placed alongside the trunk. They found a 10% incidence of upper extremity nerve injury that was not influenced by internal mammary artery harvest, internal jugular vein catheterization, or left arm position. Surgical manipulation was more contributory than extremity positioning in producing trauma to the brachial plexus. Jellish et al.12 reported that there is less slowing of somatosensory evoked potentials (SSEPs) of the ulnar nerve during sternotomy when both arms are abducted instead of tucked at the sides. However, they found no differences in perioperative symptoms between patients in the arm-abducted versus arm-at-side groups.
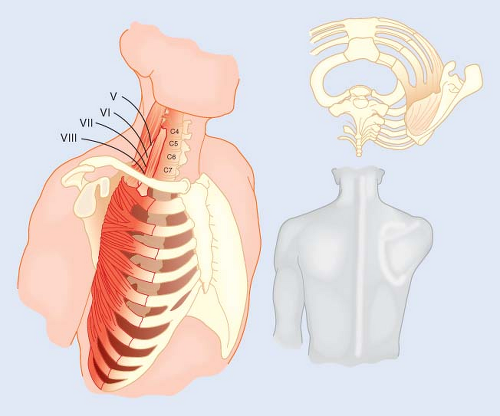
Long Thoracic Nerve Dysfunction
A number of lawsuits have centered on postoperative serratus anterior muscle dysfunction and winging of the scapula (Fig. 28-7) alleged to be the result of position-related injuries to the long thoracic nerve, a nerve arising from nerve roots C5, C6, and C7. Because C5 and C6 fibers of the nerve course through the middle scalene muscle and emerge from its lateral border to join the fibers from C7, it has been proposed that neuropathies of the long thoracic nerve are traumatic in origin.16 Because the nerve is not routinely involved in a stretch injury of the brachial plexus and because the plexus is not routinely involved when long thoracic nerve dysfunction occurs, the relationship between postoperative long thoracic neuropathy and patient positioning remains speculative. Based on the evidence of Foo and Swann17 plus data from various litigations, Martin18 concluded that in the absence of demonstrable trauma, postoperative dysfunctions of the long thoracic nerve were quite likely the result of coincidental neuropathies, possibly of viral origin.
Axillary Trauma Associated with the Humeral Head
Abduction of the arm on an arm board to >90 degrees may thrust the head of the humerus into the axillary neurovascular bundle. This bundle typically lies on the flexion side of the shoulder joint. However, when the arm is abducted to >90 degrees, the bundle is now on the extension side of the joint. The bundle is both compressed and stretched at that point, and its neural structures may be damaged. In the same manner, vessels can be compressed or occluded and perfusion of the extremity can be jeopardized.
Radial Nerve Compression
The radial nerve, arising from roots C6 to C8 and T1, passes dorsolaterally around the middle and lower portions of the humerus
in the musculospiral groove. At a point on the lateral aspect of the arm, approximately three fingerbreadths proximal to the lateral epicondyle of the humerus, the nerve can be compressed against the underlying bone and injured. Pressure from the vertical bar of an anesthesia screen or a similar device against the lateral aspect of the arm, excessive cycling of an automatic blood pressure cuff, and compression at the midhumerus level by restrictive sheets or towels used to tuck the arms have been implicated in causing damage to the radial nerve. Other support devices, including arm boards and slings used when patients are positioned laterally, can directly compress the radial nerve as it wraps around the musculospiral groove.
in the musculospiral groove. At a point on the lateral aspect of the arm, approximately three fingerbreadths proximal to the lateral epicondyle of the humerus, the nerve can be compressed against the underlying bone and injured. Pressure from the vertical bar of an anesthesia screen or a similar device against the lateral aspect of the arm, excessive cycling of an automatic blood pressure cuff, and compression at the midhumerus level by restrictive sheets or towels used to tuck the arms have been implicated in causing damage to the radial nerve. Other support devices, including arm boards and slings used when patients are positioned laterally, can directly compress the radial nerve as it wraps around the musculospiral groove.
Median Nerve Dysfunction
Isolated perioperative injuries to the median nerve are uncommon and the mechanism is usually obscure.19,20 A potential source of injury is iatrogenic trauma to the nerve during access to vessels in the antecubital fossa, as might occur during venipuncture. Anecdotally, this problem appears to occur primarily in men 20 to 40 years of age who cannot easily extend their elbows completely. Forced elbow extension after administration of muscle relaxants and while positioning the arms, with resultant stretch of the median nerve, has been suggested as one potential mechanism for this problem.
Ulnar Neuropathy
Improper anesthetic care and patient malpositioning have been implicated as causative factors in the development of ulnar neuropathies since reports by Büdinger21 and Garriques22 in the 1890s. These factors likely play an etiologic role for this problem in some surgical patients. Other factors, however, may contribute to the development of postoperative ulnar neuropathies. In a series of 12 inpatients with newly acquired ulnar neuropathy, Wadsworth and Williams23 determined that external compression of an ulnar nerve during surgery was a factor in only two patients. Ulnar neuropathies develop in medical as well as surgical patients.24 The mechanisms of ulnar neuropathy are unclear.
Typically, anesthesia-related ulnar nerve injury is thought to be associated with external nerve compression or stretch caused by malpositioning during the intraoperative period. Although this implication may be true for some patients, three findings suggest that other factors may contribute. First, patient characteristics (e.g., male sex, high body mass index, and prolonged postoperative bed rest) are associated with these ulnar neuropathies.25 Various reports suggest that 70% to 90% of patients who have this problem are men.19,20,23,24,25 Second, many patients with perioperative ulnar neuropathies have a high frequency of contralateral ulnar nerve conduction dysfunction.26 This finding suggests that many of these patients likely have asymptomatic but abnormal ulnar nerves before their anesthetics, and these abnormal nerves may become symptomatic during the perioperative period. Finally, many patients do not notice or complain of ulnar nerve symptoms until >48 hours after their surgical procedures.25,26 A prospective study of ulnar neuropathy in 1,502 surgical patients found that none of the patients had symptoms of the neuropathy during the first 2 postoperative days.27 It is not clear whether the onset of symptoms indicates the time that an injury has occurred to the nerve. Prielipp et al.28 found that 8 of 15 awake volunteers who had notable alterations in their ulnar nerve SSEP signals from direct ulnar nerve pressure did not perceive a paresthesia, even when the SSEP waveforms decreased as much as 72%.
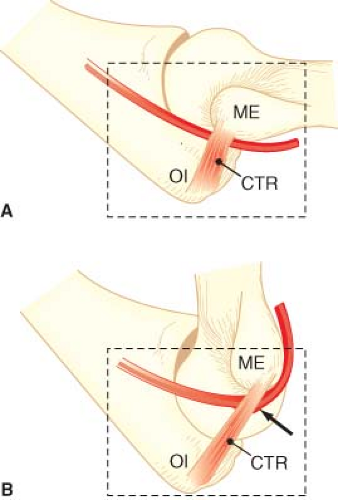
Elbow flexion can cause ulnar nerve damage by several mechanisms. In some patients, the ulnar nerve is compressed by the aponeurosis of the flexor carpi ulnaris muscle and cubital tunnel retinaculum when the elbow is flexed by >110 degrees29,30 (Fig. 28-8). In other patients, this fibrotendinous roof of the cubital tunnel is poorly formed and can lead to anterior subluxation or dislocation of the ulnar nerve over the medial epicondyle of the humerus during elbow flexion. This displacement has been observed in approximately 16% of cadavers in whom the flexor muscle aponeurosis and supporting tissues have not been dissected.31,32 Ashenhurst32 has speculated that the ulnar nerve may be chronically damaged by recurrent mechanical trauma as the nerve is in subluxation over the medial epicondyle.
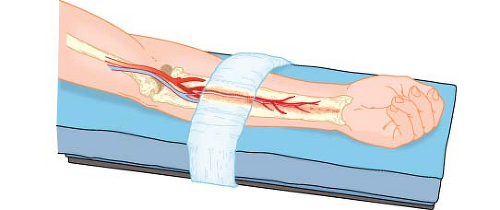 Figure 28.9. Arm restraint, if excessively tight, can compress the anterior interosseous nerve and vessel against the interosseous membrane in the volar forearm to produce an ischemic neuropathy. Reproduced from: McLeskey CH, ed. Geriatric Anesthesiology. Baltimore, MD: Williams & Wilkins; 1997:155, with permission.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|