CONTENTS
3-in-1 Solutions or Total Nutrient Admixtures
Micronutrients and Electrolytes
Monovalent Anions and Cations Balancing
Ion Balancing Example K, Na, Cl, and OAc
Ion Balancing Example K, Na, Cl, OAc, and PO4
Additional Considerations Based on Formulations
Chloride and Acetate: Cl and OAc
Magnesium: Mg (Generally Ordered as MgSO4)
Calcium: Ca (Preferred as Ca Gluconate)
Limits and Guidelines for Stability and Solubility
Common Additives to PN in the Pharmacy
Common Additives to PN in the Home
Medications Not to Be Administered While PN Infusing
Custom Formulations versus Premixed Formulations
Selected References and Resources
Parenteral nutrition (PN) can be a life-sustaining and life-enhancing therapy but does not come without significant risks. Safe administration of PN requires a solution that is sterile, stable with components that are compatible in solution, in addition to being clinically appropriate. A solution may be proposed that has the carefully determined components to meet the patient’s needs but these components must be able to safely form a stable solution to be administered. This chapter focuses on how to determine if a proposed clinically appropriate formulation can be safely compounded and administered.
PN is essentially a combination of electrolytes, macro- and micronutrients, and trace elements mixed into a single solution instead of being administered separately to allow for an effective administration of nutrition support. Medications that are compatible with the solution may also be incorporated into the PN formulation. In many cases, especially in the outpatient or home environment, a patient receiving PN is receiving a custom mixture designed specifically to meet their needs based on the individual’s electrolyte levels, nutritional needs, and medical history. Because each solution is unique and customized to the patient, the stability and the compatibility of each component and the entire mixture must be evaluated on an individual basis with each change.
In order to understand this concept, a good working definition of stability, sterility, and compatibility is necessary. Compatibility is best explained as the type and amount of an ingredient that can safely be combined with other components into a PN solution. Many different ingredients can be placed in a PN; however, the “safe” concentration of the ingredient may vary with the agent, the salt form of the ingredient, and the concentration of the other components in the solution. Calcium and phosphate are the best-known examples of concentration-based compatibility. Other factors that affect compatibility are pH, temperature, and order of mixing of the ingredients.
Stability is best defined as how well all the ingredients are able to remain compatible. In general, stability is assessed with a product maintaining the desired activity within 10% of original levels. The same factors that impact compatibility also impact the stability of the solution. Additionally, over time, a product may slowly degrade in a solution and result in either insufficient activity of the product or a new opportunity to interact with another product due to molecular changes from degradation. A solution may be compatible in the short term but maintaining that compatibility over a minimum of the storage period plus infusion period is required for a solution to be determined to be stable.
MACRONUTRIENT COMPATIBILITY
Macronutrient compatibility is often of limited concern for the amino acid, dextrose, and water components. Most clinically suitable concentrations are compatible for the commercially available amino acid products. The stability of these products can far exceed the sterility limitations of a compounded product. Commercially compounded products are often stabile for a period of years in clinically suitable ready-to-administer solutions. General compatibility guidelines for PN macronutrients in solution to ensure 9 days refrigerated and 24 h room temperature stability would be as follows:
Dextrose | ≥10% |
Amino acid | ≥4% |
Fat emulsion | ≥2% final concentration (more than 2 g fat emulsion/100 mL or 20 g/L) |
The greatest concern that does arise with macronutrients comes from environmental influences on longer-term stability with both the concentrated bulk solutions and ready-to-use (RTU) formulations. This is not due to compatibility with other macronutrients but in the solution itself maintaining stability in varying environments. Extremes in temperature, exposure to light, and changes in humidity can have significant impact on the macronutrients, resulting in oxidation of lipid products and denaturing of proteins. The denaturing of a protein is extremely dangerous as this breaks the protein into foreign proteins, which may no longer be physiologically acceptable to the human body. These foreign proteins can cause significant systemic reactions. It is important to note that the solution may appear visibly unchanged and therefore monitoring of environmental conditions and education of patients at home regarding proper handling of solutions is critical in preventing this occurrence.
The impact of the environmental factors is more significant for the outpatient than in an inpatient acute care setting. In general, the outpatient environment is less monitored, more difficult to control, and less stable. Individual patients prefer different environmental conditions and this is more customized in the outpatient setting where one home may have a very different climate than another and both considered “room temperature.” Additionally, fewer patients monitor their refrigerator temperatures and may use less reliable appliances that create uneven cooling. Patient’s lifestyle may impact the temperature extremes to which the solution is exposed. The actual dispensing and delivery of the product is also different as generally multiple units are made at one time and stored by the clinic, patient’s home, or long-term care facility outside the monitoring of the pharmacy and the solutions may undergo shipping and handling extremes during transit. All of these factors can cause a greater risk to stability for the same solution for the outpatient versus the inpatient.
MACRONUTRIENTS
Macronutrient compatibility can vary based on the pH of the base solution as determined by the concentration and components of the solution. Amino acid solutions are the main drivers of the pH of the PN solution. Amino acids have similar pH (5.2–6.5 is the range of the commonly used products). Because of the concentration of bulk amino acids (maximum of 20% vs. the concentration of dextrose 70%), amino acids are commonly the primary macronutrient component of the PN solution. Therefore, the overall pH of the PN solution is generally governed by the amino acid component. While there are no true clinical studies that validate this, one can look at the calcium–phosphate solubility curves published by the amino acid manufacturers and observe that the higher the amino acid and dextrose concentration of the test solution, the more calcium and phosphate that can be safely added to the solution. Thus, there is a relationship between the pH of the PN solution, and the lower the pH, the better the calcium–phosphate solubility.
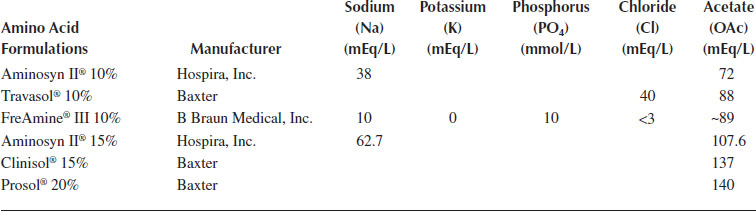
Inherents—Every amino acid solution has some amount of sodium, potassium, chloride, and acetate. These are commonly referred to as the “inherents” or inherent cations and anions and are electrolytes used to buffer and stabilize the solution. Many prescribers do not address these since they are actually part of the amino acid solution and cannot be changed. Other prescribers will include the inherents in the total amount of electrolytes to be provided to the patient. The exact amount varies between products and can be found in the package insert provided by the manufacturer for each product (Table 11.1). The pharmacist should verify with the prescriber or pharmacy that is making the PN solution if inherents need to be taken into consideration when determining the amount of electrolytes added to each PN solution compounded.
FreAmine® (B Braun, California) is the exception because it includes phosphorous and not potassium as inherents. Like other amino acids, it has inherent sodium, chloride, and acetate. Unlike other amino acids, it also has phosphorus but does not have potassium. In the early days of PN, patients would become hypophosphatemic because the PN solutions did not contain sufficient amounts of phosphorous. In order to combat this issue, B Braun, the original manufacturer of FreAmine, began adding phosphorus to their amino acid formulation. Each liter of FreAmine 10% contains 10 mmol phosphorus.
Inherents in Common Amino Acid Formulations
SPECIALTY AMINO ACIDS
Certain patient populations require more customized amino acid profiles to optimize PN therapy (Table 11.2). While there is some controversy over the value of specialty amino acids, for liver or kidney disease, there is much less controversy over the use of specialty amino acids for premature infants and very young children. The debate here is related to the duration of use. The neonatal specialty amino acids are designed to provide higher concentrations of amino acids needed in early childhood development. They contain cysteine, which is necessary for growth and development and has been found to increase the calcium–phosphate solubility that is very important in the young child population. The manufacturers have published solubility curves; however, the curves cannot be interchanged. If a precipitate forms, the entire solution and tubing should be disposed of immediately.
3-IN-1 SOLUTIONS OR TOTAL NUTRIENT ADMIXTURES
A significant concern arises when a “3-in-1” or “total” nutrient admixture in which an emulsion is formed with dextrose, amino acid, and lipid is being combined in a single solution. This emulsion can be very fragile. The ability to maintain a safe emulsion can be influenced by concentration of bases, the impact of micronutrients, temperature, light exposure, and even the handling of the product by the patient. An early “cracking” of the emulsion (separation of the lipid from the nonlipid component) can be difficult to identify, but once cracked, the solution is no longer considered stabile and not safe to administer. A cracked emulsion should never be agitated with a goal of reemulsification as the solution is already damaged.
The stability of the emulsion is significantly impacted by the activity and concentration of the other components of the solution. The exact conditions that cause emulsion cracking are not well understood and opinions vary greatly as to how to ensure a safe solution. Maintaining an appropriate concentration range and limiting the impact of multivalent ions on the fat cell walls will allow for the highest level of reliable stability. The micelle membrane of the fat molecule has an attraction to other fat molecules and this attraction allows the emulsion to stay evenly distributed and thus maintain a smooth homogenous emulsion. When this attraction undergoes stress from competing attractions, the molecules may pull apart or stick together and crack the emulsion. The higher the valence of the molecule (e.g., trivalent iron molecules), the stronger this attraction becomes and the greater the risk for emulsion instability. Observational literature has demonstrated that the very low (less than 2%) concentrations of lipids can cause the molecules to be pulled too far apart in solution, causing an uneven distribution or large variance in particle size. Therefore, this guideline is used by most clinicians as an indicator to increase caution when evaluating solution stability closer. There are multiple opinions as other factors with lipid emulsion stability, including particle size and solution components, that require further research.
Specialty Amino Acids
Specialty Amino Acid | Content | Indication/Contraindication |
Hepatamine®—B Braun Medical, Inc. | High in branched-chain amino acids (36%) leucine, isoleucine, and valine | Specifically for the treatment of hepatic encephalopathy in patients with cirrhosis or hepatitis Contraindicated in patient with anuria, inborn errors of metabolism Note: Verify that all documentation is available for reimbursement purposes as Hepatamine is billed using different codes than traditional amino acids |
Nephramine®—B Braun Medical, Inc. | Contains only essential amino acids in a 5.4% solution | Indicated in patient with chronic renal failure |
Premasol®—Baxter | 6% and 10% amino acid solution | For neonatal and pediatric use only |
Trophamine®—B Braun Medical, Inc. | 6% and 10% amino acid solution | Better calcium and phosphate solubility than Premasol due to differences in the manufacturing process as reflected on the manufacturers’ solubility curves |
MICRONUTRIENTS AND ELECTROLYTES
Electrolyte additives may be ordered in many different ways although they can only be added as a salt since an ion cannot exist alone but will always bind to another ion in the environment to be electrically neutral then separate and rebind to other ions in solutions. However, ordering is not always simple. Electrolytes may be ordered as salts, or as ions, as mEq, mmol, mg of elemental Ca, for example, per bag, per day, per L, per 100 mL, or per kg. The person ordering and compounding the PN must ensure they know exactly what the desired outcome is for the formula. Owing to the risks associated with “misunderstanding” the PN order and possible impact on patient safety, the American Society of Parenteral and Enteral Nutrition (A.S.P.E.N.) has created safety guidelines for ordering PN solutions. These guidelines can be found on the A.S.P.E.N. website (www.nutritioncare.org) and should be reviewed often to minimize the risk of patient harm from misreading a PN order.
MONOVALENT ANIONS AND CATIONS BALANCING
Monovalent anions and cations include sodium, potassium, chloride, and acetate. Additionally, phosphorus presents as phosphate, which is a monovalent compound. All of these must match in quantity to create an electrically neutral solution. In order to balance a solution, one will determine the amount of Na and K ordered. Since phosphorus requires a monovalent anion to form a dissolvable salt, first determine the amount of Na or K that will be used in providing the phosphate. The remaining Na and K will be provided combined with acetate and chloride.
• NaPO4 = 4 mEq Na and 3 mmol PO4/mL
• KPO4 = 4.4 mEq K and 3 mmol PO4/mL
Note: Some prescribers and some facilities include the “inherent” cations and anions that are part of the amino acid solutions. In this case, these will be calculated and subtracted from the order prior to “balancing” the formula. It is important to know if this is included in the order and be consistent in calculations. For the following examples, one will assume the inherents either are not included in the order or have already been subtracted.
A desired ratio should be noted on the order or it is assumed to be balanced as 1:1. Shifting this balance can shift the overall pH of the formula as well as affect the patient’s acid–base balance and thus the lab values seen for CO2 and Cl−. Once this ratio is determined, the chloride (Cl) and acetate (OAc) salts can be assigned. It is a best practice to reduce the number of ingredients and create the formula with the least number of salts.
ION BALANCING EXAMPLE K, NA, CL, AND OAC
The PN orders say 40 mEq K+ and 120 mEq Na+. Cl− to OAc− 1:1
K+ (40) + Na+ (120) = 160+ charges. Need 160− charges (80 as Cl− and 80 as OAc−)
In the PN, the following could be added: KCl 40 mEq, NaCl 40 mEq, and NaOAc 80 mEq. (There are additional options available for each formula presented based on salts available. This is one example for demonstration purposes.)
The PN orders say 40 mEq K+ and 120 mEq Na+. Cl to acetate 3:1.
K+ (40) + Na+ (120) = 160+ charges. Need 160– charges: (120 Cl− and 40 OAc−)
In the PN, KOAc 40 mEq, NaCl 120 mEq could be added
or
In the PN, KCl 40 mEq, NaCl 80 mEq, and NaOAc 40 mEq added depending on compounder choice and availability of salts. The second option is less desirable due to increased number of additives.
ION BALANCING EXAMPLE K, NA, CL, OAC, AND PO4
The PN orders say 40 mEq K+ and 120 mEq Na+. Cl− to OAc− 1:1 and 15 mmol PO4 (the amino acid is not FreAmine)
K+ (40) + Na+ (120) = 160+ charges. Need 160– charges (15 mmol PO4−, 50% remainder as Cl− and 50% as OAc−)
The PN could have an additional KCl 40 mEq, NaPO4 15 mmol (representing 20 Na+ charges and thus 20 negative charges since every 4 mEq of Na for every 3 mmol of PO4 as noted above) and NaCl 30 mEq and NaOAc 70 mEq. Thus, the formula has the 40 of K+, 120 of Na+ balanced with the 15 mmol of phosphate, 70 mEq of Cl−, and 70 mEq of OAc−
ADDITIONAL CONSIDERATIONS BASED ON FORMULATIONS
SODIUM: NA
• Avoid exceeding 154 mEq/L. When administering hypertonic solutions, undesired fluid and electrolyte shifts may occur between extracellular and intracellular fluids.
POTASSIUM: K
• The primary limitation is administration rate, which should not exceed 10 mEq/h in the absence of cardiac monitoring.
CHLORIDE AND ACETATE: CL AND OAC
• Both may influence the pH of the solution, leading to instability. If provided in excess, it may also impact the pH of the patient.
• In cases of low CO2, sodium bicarbonate should not be added to the PN due to stability concerns.
PHOSPHORUS: PO4
• Generally ordered in mmol of phosphate (1 mmol of phosphate = 31 mg elemental phosphorous).
• When receiving a PN order, always verify the units the PO4 is ordered in. Some institutions may order as mg phosphorus or mEq. This is a significant opportunity for medication error prevention. If unsure, always verify with the prescriber.
• If FreAmine is the amino acid, allow for the inherent PO4 in FreAmine.
• When compounding, PO4 is generally added to the PN solution by hand at the very end of the compounding process to ensure maximum volume and solubility of the solution.
MAGNESIUM: MG (GENERALLY ORDERED AS MGSO4)
• Divalent cation.
• Verify units used in the ordering of Mg. Some hospitals may order in mg of Mg, mg MgSO4, or mEq MgSO4. This is a potential area for medication errors.
CALCIUM: CA (PREFERRED AS CA GLUCONATE)
• Divalent cation.
• Verify units used in the ordering of Ca. Some hospitals may order in mg of elemental Ca, mg of the Ca salt, or mEq Ca gluconate. This is a potential area for medication errors.
• In emergency cases of shortages, CaCl2 can be used. If CaCl2 is used, patient safety is a major concern due to changes in the overall solubility of the solution. Of note, if the chloride salt is being used, the sulfate salt for magnesium should be discouraged to avoid the formation of calcium sulfate in the solution which may pass through a traditional PN tubing filter.
• Although this can also occur when magnesium is added to other calcium-containing solutions, the calcium chloride salt is more likely to separate in solution and result in this insoluble compound. The microprecipitates formed are too small to be filtered out using the typical filters and can silently accumulate in the body over time, causing injury.
OTHER ADDITIVES
• Iron
• Iron has a significant impact on lipids due to being a trivalent cation, and immediately separates from its carbohydrate carrier once in the presence of the active amino acid solution and then the ion affects the fat cell wall.
• Be aware of its presence in some trace element formulations. At the time of this publication, the only iron product studied with PN is iron dextran.
• Other iron salts may be administered to patients receiving PN, but should not be added to the PN solution or infused via the PN dedicated line.
• Cysteine
• Not actually a direct impact of cysteine but an indirect impact of the salt formulation from the HCl acid molecule tied to the cysteine (see discussion below).
• Since nutritional requirements for additional cysteine are limited, the cysteine may get removed at a certain age but then the formulation must be reevaluated. This pH shift creates changes that may affect stability.
• Zinc
• Solubility is concentration and pH dependent; current literature does not recommend a total of more than 10 mg/L in any PN solution.
• Individual trace elements should be added at the time of mixing. Current literature should be reviewed prior to adding individual trace elements and additions should be based on patient’s needs and disease state.
LIMITS AND GUIDELINES FOR STABILITY AND SOLUBILITY
The solubility of a solution is a concentration- and pH-dependent interaction. The pH of the solution (as determined by the concentration of the macronutrients and the additives) is the main determinant of the solubility. Additional influence comes from temperature, so a solution that is soluble at room temperature may not be as the formula is heated or cooled. The order in which the products are added into the solution can also impact solubility. The shaking of a solution can break the amino acids and thus impact pH and create instability.
There are very limited guidelines for stability and solubility and many are anecdotal. Additionally, the information that is published in standard reference guides such as Trissel’s Handbook of Injectable Drugs©, Extended Stability for Parenteral Drugs©, or King Guide© is from single solutions that are tested. Therefore, the data are specifically related to that formula and the information should be extrapolated to other solutions carefully.
Specific additives and medications can also impact solubility. Certain medications will precipitate in the PN solution or cause amino acids or additives to become insoluble through changes in pH or direct binding. Calcium and phosphorus are one of the most common causes of solution instability and precipitation and the reference solubility curves provided by the manufacturer should be extrapolated carefully.
EXAMPLES OF COMMON CAUSES OF PRECIPITATION OR SOLUTION INSTABILITY
• Using the incorrect amino acid curve or incorrect units (mg/mmol/mEq)
• Adding an additive that impacts the pH
• Too high of a concentration of acetate
• Using a traditional amino acid, but the solubility curve from Trophamine or Premasol
• Using a different salt
• Calcium chloride instead of calcium gluconate
• Dispensing or compounding in a smaller volume such as pooling or using dual-chamber bags
• Adding calcium and phosphorus too close together or in too small of a volume. Examples of this include
• Adding both calcium and phosphorus to a pool solution instead of adding second to the diluted final volume
• Dispensing in a dual-chamber bag but calculating solubility based on the final volume
• Not agitating solution between ingredients to ensure distribution in solution
• Improper compounding temperatures (exceeding United States Pharmacopeia [USP] guidelines) or storage conditions. The presence of a light source, such as bili-lamps or prolonged exposure to sunlight, may lead to solution instability
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





