Acute pain: 0 to 4–6 wks
Subacute pain: 1 to 3 mos
Chronic pain: Persistent pain >3 mos without biologic value
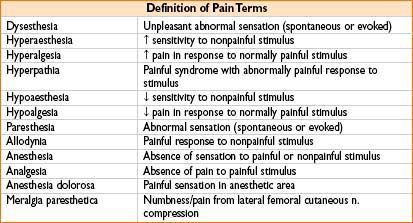
Figure 29-1. Algorithm for pharmacologic management of chronic pain.
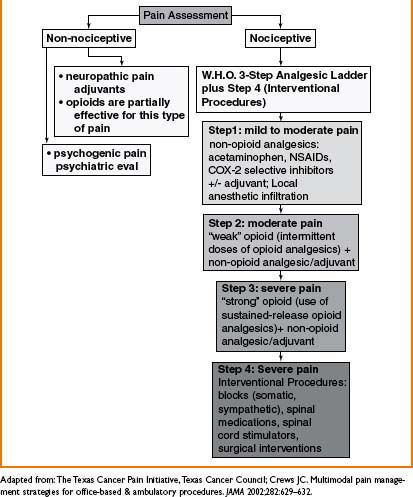
LOW BACK PAIN (LBP)
• Annual incidence: 15% of population; 60–85% lifetime incidence; costs: >$90 billion/year
• Risk factors: Smoking, obesity, older age, female gender, physical labor, sedentary lifestyle, low educational achievement, worker’s compensation insurance, psychological factors (e.g., anxiety, depression, somatization disorder)
• Prognosis: 90% spontaneously recover within 4–6 wks; after 6 months <50% return to work; after 1 yr only 10% return to work

Low Back Pain Evaluation
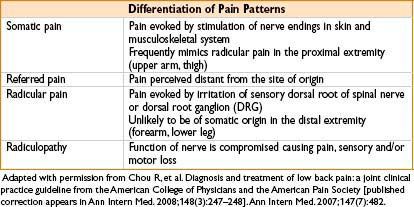
Rule out emergencies (e.g., infection, hematoma, acute neurologic deterioration, tumor progression)
• History
• Inciting factors/trauma, quality, onset, location, radiation, intensity, frequency, duration, exacerbating/alleviating factors (e.g., meds, rest, physical therapy), h/o back pain, h/o malignancy or constitutional symptoms (fever, weight loss), pain worse at night, bowel/bladder dysfx, sexual dysfx, h/o peripheral vascular dz or abd aortic aneurysm, pending legal proceedings, depression/anxiety, prev back pain evals (office/ER visits, imaging studies), prior treatments (e.g., noninterventional and interventional)
• Physical examination
• Visually inspect spine: Scoliosis, deformities, local skin changes (e.g., suggestive of infection, vascular disease, or complex regional pain syndrome)
• Palpate for tenderness (over spinous processes and paraspinal)
• Assess range of motion in extension/flexion/lateral rotation of spine (pain with flexion may reflect disc dz, pain with extension may reflect facet arthropathy or spinal stenosis, pain with lateral motion may reflect disc disease/herniation or spondylosis)
• Ask if any movements reproduce or alleviate pain; evaluate gait & hip joint
• Rectal, abd, & pelvic examinations to rule out pathology in prostate, bladder, abd, & pelvis
• Perform provocative tests (e.g., straight leg raise, Faber’s and Gaenslen’s test)
• Psychosocial evaluation (e.g., SOAP, COMM, DAST)
• Diagnostic Imaging (e.g., plain film radiography, CT, MRI, SPECT, provocative discography, myelography, ultrasound)
• Additional diagnostic testing (e.g., electrodiagnostic testing (EMG/NCV), bony vibration test, interventional procedures (e.g., facet medial branch block [FMBB], selective nerve root block [SNRB]))
• Laboratory testing (usually not necessary unless suspect infx, malignancy or rheumatologic dz)
Initial Evaluation
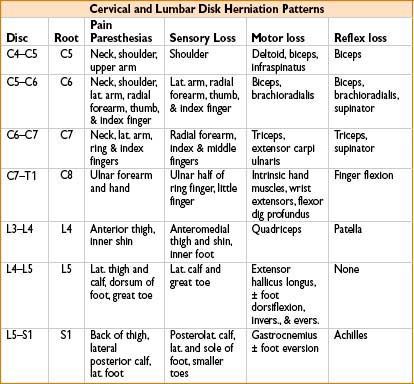
Strength Testing

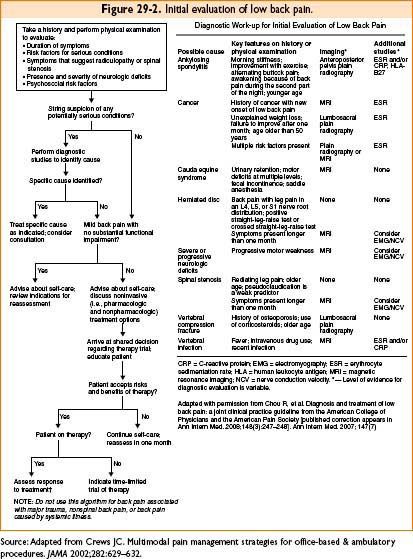
Sensory Testing
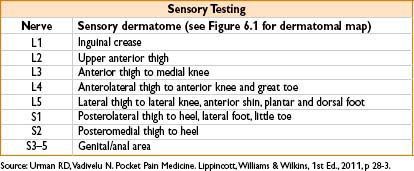
Reflex Testing
Patellar reflex: L3-4 innervation
Achilles reflex: S1-2 innervation

Low Back Pain Treatment
• Noninterventional therapy:
• Nonpharmacologic therapy (physical therapy, stretching and strengthening exercises, massage, mind/body exercises, home exercise program, relaxation therapy, biofeedback (assistance with modification of pain perception), acupuncture, TENS, lifestyle modification (smoking cessation), avoidance of triggers, ice, heat, cognitive-behavioral therapy)
• Pharmacologic therapy (analgesics and adjuvants):
• NSAIDs, acetaminophen, muscle relaxants (cyclobenzaprine), tricyclic antidepressants (nortriptyline), selective serotonin reuptake inhibitors (sertraline, fluoxetine), serotonin norepinephrine reuptake inhibitors (duloxetine), anticonvulsants (gabapentin), local anesthetics (lidocaine patch), opioids
• Interventional therapy:
• Epidural/caudal steroid injection (ESI/CSI), SNRB (selective nerve root block), facet joint injection (FJI), FMBB (facet medial branch block), radiofrequency lesioning (RFL), nerve rhizotomy, spinal cord stimulator (SCS), intrathecal therapy, chemonucleolysis, intradiscal ablative procedures, disc replacement therapy with spinal motion-sparing technology
• Can provide significant pain relief but are often temporary and require repeat procedures
• Some procedures can be performed as diagnostic tests (facet injections, FMBBs)
• SCSs can be especially helpful in the treatment of extremity pain
• Surgical procedures (e.g., discectomy, laminectomy, spinal fusion)
• Indications: Acute evacuation of hematoma, acute cauda equina syndrome, progressive/severe neuromotor deficit, persistent neuromotor deficit after 6–8 wks of treatment, infection, trauma/fracture, tumor, severe anatomic deformities
Interventional procedures should be avoided if the patient is on anticoagulants, has a coagulopathy or infection at the site of the injection. Risks include inadvertent intraneural, epidural, intrathecal, subdural, and intravascular injection.


Acute low back pain treatment:
• 4 to 6 weeks since onset
• 90% of all patients with back pain have resolution within 6 weeks.
• Must rule out more serious conditions (see “Red Flags”)
• Diagnostic imaging (consider plain film radiography, CT, MRI, depending on severity and urgency of symptoms)
• Goal is to return to normal lifestyle quickly.
• Usually responds to noninvasive treatment:
• Set expectations, patient education, self care
• Modification of current activity level, gentle physical therapy
• Develop home exercise program
• Heat or ice
• Pharmacologic therapy (NSAIDS, acetaminophen, muscle relaxants, usually does not require opioid therapy)
Subacute low back pain treatment:
• 4–6 weeks to 6 months since onset
• Goal is to slow and/or stop progression to chronic back pain with return to normal lifestyle
• Diagnostic imaging (plain film radiography, CT, MRI depending on severity and urgency of symptoms).
• Physical therapy, and also develop home exercise program
• Consider referral to spine/pain specialist, psychosocial evaluation
• Nonemergent radiculopathy: consider surgical evaluation and minimally invasive interventional procedures (e.g., epidural steroid injections)
• NSAIDS, acetaminophen, muscle relaxants, antidepressants, +/– opioids
Chronic low back pain treatment:
• >6 months since onset
• Detailed history and physical examination
• Goal is to limit symptoms with return to modified lifestyle.
• Diagnostic imaging (plain film radiography, CT, MRI repeat imaging if necessary; e.g., new onset or change in symptoms)
• Physical therapy, and also develop home exercise program
• Lifestyle modifications as necessary
• Referral to spine/pain specialist (for interventional procedures), psychosocial evaluation
• Long-term treatment: noninvasive and invasive therapy (see treatment section above)
LUMBAR SPINAL CANAL STENOSIS
Pathophysiology
• Degeneration of discs, joints, and/or vertebrae lead to instability causing osteophyte formation, hypertrophy, and calcification of the posterior longitudinal ligament, leading to narrowing of the spinal canal (central +/- or lateral recess)
• May result either in central canal and/or foraminal stenosis
• Stenosis may stabilize or progress
• Symptoms also known as neurogenic claudication
Evaluation
• History and physical examination
• Examination can be relatively normal; result of imaging studies important for diagnosis
• Constant or intermittent low back pain, radiates to one/both lower extremities
• Often, patients walk leaning forward (“shopping cart posture”)
• Loss of lumbar lordosis
• Pain mainly perceived with walking, especially downhill and/or standing
• Neurogenic claudication (bilateral posterior leg pain worsening with ambulation, relieved with sitting and leaning forward)
• Central stenosis: Axial and radicular pain, radicular pain usually nondermatomal (e.g., lower extremities ache with walking); otherwise normal lower extremity neurologic examination
• Foraminal stenosis (can cause radicular pain): may present with sensory, motor, or reflex changes along dermatomal distribution; exacerbating factors: Walking (especially downstairs), standing, extension (buckling of ligamentum flavum and disc protrusion), relieving factors: Rest, sitting/lying down, flexion
• Diagnostic imaging: Very important for diagnosis
• CT: Visualization of bony and ligamentous changes
• MRI: Visualization of spinal cord and nerve root involvement
Treatment
• Noninterventional therapy: Refer to treatment section in introduction
• Interventional therapy:
• Central canal stenosis: Interlaminar/transforaminal epidural (if single level stenosis)/caudal steroid injection (if multilevel stenosis) caudal steroid injection
• Foraminal stenosis: Epidural steroid injection, SNRB
• Interspinous devices (e.g., X-stop to decrease extension, disc compression with milder stenosis)
• MILD procedure (percutaneous lumbar decompression)
• Intrathecal therapy (esp beneficial for spinal stenosis due to compression fracture or after failed surgical intervention)
• SCS (thoracic and lumbar MRI to evaluate room for lead placement), DRG stimulation (for central canal stenosis), paddle leads (if no room for percutaneous leads)
• Surgical procedures (e.g., decompression surgery)
FAILED BACK SURGERY SYNDROME
Pathophysiology
• Diagnosis of failed back surgery syndrome is not a specific diagnosis, per se, and is discouraged from use in lieu of a more specific diagnosis.
• Causes: scar tissue in the spinal canal, development of adhesions, nerve injury during surgery, recurrent disc herniation, facet joint arthropathy above/below the area of fusion, instrumentation irritation of nerves/bone, CRPS
• Persistence or recurrence of original pain or development of new pain.
Evaluation
• History and physical examination:
• History of lumbar surgery (obtain pre- and postoperative symptoms, operative findings and levels of instrumentation are important to evaluate new findings)
• Diagnostic imaging:
• Plain film radiography: evaluate bony/instrument changes.
• CT: evaluate bone and canal space in setting of MRI incompatible hardware.
• MRI: evaluate soft tissue in setting of MRI-compatible hardware or after surgeries without placement of hardware.
Treatment
• Difficult to treat
• Noninterventional therapy: refer to treatment section in introduction.
• Interventional therapy:
• Epidural injections: may not reach affected areas that are blocked by scar tissue or adhesions
• Paravertebral/transforaminal injections possibly may bypass the scar tissue to directly affect the involved nerve.
• Epiduroscopy with lysis of adhesions (no longer commonly performed).
• Intrathecal therapy (often limited effictiveness)
• Spinal cord stimulator may be useful especially for radicular pain. (some evidence that possibly better than medical management and reoperation).
• Other: DRG (dorsal root ganglion), PNF (peripheral nerve field) and DB (deep brain) stimulation are possible
• Surgical procedures: repeat surgery is typically not successful
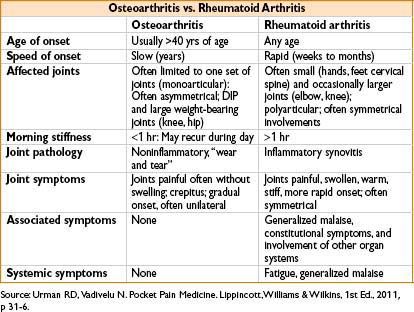
Osteoarthritis vs. Rheumatoid Arthritis
PERIPHERAL NEUROPATHIC PAIN
• Causes include diabetes, cancer, herpes zoster, infections, trauma, autoimmune dz
• Injury to peripheral nerves are often associated with autonomic nerve disorders
Diabetic Neuropathy
Pathophysiology
• From diabetic microvascular injury involving small blood vessels that supply nerves
• Associated with sensory, motor & autonomic symptoms
• Often associated with 3rd third nerve palsy; mononeuropathy; mononeuropathy multiplex; diabetic amyotrophy; polyneuropathy; autonomic and thoracoabdominal neuropathy
Symptoms
• Sensory: Burning, pricking, pins-and-needle, shooting, electric sensation
• Motor: Distal impaired fine coordination & proximal weakness (difficulty climbing stairs)
• Autonomic: Often involving the cardiovascular, GI and/or genitourinary system
Evaluation
• Assessment of the appearance of the feet, presence of ulceration, and ankle reflexes
• Normal results on vibration testing (128 Hz tuning fork) or monofilament make large fiber peripheral neuropathy from diabetes less likely
• Nerve conduction tests may show ↓ peripheral nerve fx, but not appropriate routine test
Treatment
• Mostly preventive & symptomatic measures
• Optimize glycemic control
• Pharmacologic treatment: Topical (lidoderm patches, capsaicin), Tricyclic antidepressants (amitriptyline, nortriptyline, desipramine), Selective norepinephrine reuptake inhibitors (duloxetine, venlafaxine), anticonvulsants (pregabalin)
• Physical therapy, including gait training
• Transcutaneous electrical nerve stimulation (TENS) and interferential current (IFC)
• Other (alpha-lipoic acid, methylcobalamin, c-peptide, photo energy therapy)
Postherpetic Neuralgia (PHN)
• Incidence ∼131/100,000 per year; more likely in elderly or immunocompromised patients
• Reactivation of varicella virus in ganglion → ganglionitis & segmental peripheral neuritis
Symptoms
• Most (50%) AHN and PHN occurs in the thoracic dermatomes (especially T4–T6)
• Most common sites on head/neck: V1 (10–20%) and cervical dermatomes (10–20%)
• 75% of patients with AHN have dermatomal prodromal pain (deep burning, throbbing, stabbing) that may precede the rash by days to weeks
• 3–7 d later, affected area becomes red and swollen with typical patches of small herpetic vesicles, loss of normal skin color, and corneal ulceration possible; skin lesions crust by 7–10 d and heal in approximately 1 mo
• Hyperesthesia generally resolves with healing of skin lesion
• Complications include bacterial skin infection, uveitis, keratitis, motor neuropathy, meningitis, and herpes zoster oticus
• Systemic signs of fever, stiff neck, headache, nausea, regional or diffuse adenopathy occur in 5% of cases, but are usually not associated with complications
• 10–15% of patients with AHN develop PHN, defined as pain lasting beyond 4 mos after the onset of the rash (pain is often severe, debilitating, constantly burning, and exacerbated by light touch, movement, temperature, and anxiety)
Evaluation
• History and physical examination
• Lab studies (serum IgM antibody, PCR for virus DNA, Tzanck smear for acute infection)
• Diagnostic imaging: CT or MRI (to exclude other causes)
Treatment
• Primary goal: Reduce acute pain and chances of developing PHN
• Treat within the first 72 hrs: decrease duration of symptoms and prevent PHN
• Antivirals: High-dose oral acyclovir speeds resolution of acute lesions & may reduce the risk of prolonged pain
• In immunocompromised patients, IV acyclovir can prevent disease progression
• No long-term benefit when corticosteroids added to acyclovir regimen
• Opioids: Helpful in relieving the acute aching pain
• Adjuvant analgesics: Gabapentin, antidepressants
• Carbamazepine: Rigid lab monitoring for myelosuppression is required
• Phenytoin: Rigid serum level monitoring (risk of pseudolymphoma-like state, reduction in folic acid levels, megaloblastic anemia)
• Adjunctive therapy: Zinc oxide ointment, topical aluminum sulfate
• Lifestyle and behavioral modification
• Alternative therapy (e.g., biofeedback, relaxation therapy)
• Stretching exercises of affected area
• Interventional therapy
• Stellate ganglion blockade: May provide symptomatic improvement (often combination of local anesthetic and corticosteroid) and prevent PHN
• By blocking profound sympathetic stimulation, it may prevent ischemia in the intraneural capillary bed with irreversible nerve damage
Complications
• Especially likely in elderly and immunocompromised patients: Cutaneous and visceral dissemination
• Myelitis (e.g., loss of bowel, bladder function, paresis)
• Neuropathy, stroke
• Ocular (e.g., keratitis, vision loss), hearing loss
Complex Regional Pain Syndrome (CRPS)
• Type I (reflex sympathetic dystrophy [RSD], Sudeck’s atrophy, reflex neurovascular dystrophy [RND], algoneurodystrophy) has no demonstrable nerve injury
• Type II (causalgia)
Symptoms
• Characterized by a burning, searing pain out of proportion with the inciting trauma or clinical appearance
• Reported as a sequela of trauma, surgery, difficult dental work, or head injury but there are cases that have no reported injury to the affected site
• Localized heat, redness, and pain have been noted, as well as trophic changes seen in the extremities with typical CRPS
• Pain precipitated or increased by local stimulation or stress
Evaluation
• History and physical examination
• Diagnostic imaging: CT. MRI, SPECT
Treatment
• Stretching exercises of affected area
• Pharmacologic therapy
• Antidepressants, anticonvulsants, intranasal ketamine, topical clonidine, transdermal lidocaine, mexiletine
• Second line: Opioids
• Lifestyle and behavioral modification
• Alternative therapy (e.g., biofeedback, relaxation therapy)
• Interventional therapy
• Stellate ganglion or lumbar sympathetic blockade may give symptomatic improvement (often combination of local anesthetic and corticosteroid)
• Surgical sympathectomy has been performed where blockade has given only temporary relief
• Dorsal column stimulation (SCS)
TRIGEMINAL NEURALGIA
Incidence
• ∼10,000 Americans develop trigeminal neuralgia each year
• Annual occurrence is 4/100,000
• Onset rare before age 35, usually occurs >50 yrs of age
• Female-to-male ratio is 1.7:1
• Right side involvement is more common
• Tic douloureux refers to the grimace that occurs with pain attacks
Etiology
• Most commonly idiopathic
• Microneuromas of CN V or vascular compression/pulsation (usually by superior cerebellar artery)
• Other causes include aneurysm, multiple sclerosis (MS), tumor (schwannomas of CN V, extraneural compression by tumor of surrounding tissue), trauma, chronic basal meningitis, diabetes mellitus (DM), congenital anomalies
• Note: Aneurysm, MS, tumor, trauma, DM, congenital anomalies should be accompanied by other physical signs and symptoms (symptomatic trigeminal neuralgia)
Symptoms
• One of the most severe types of pain one can suffer: Treat as emergency to prevent possible suicide
• Severe episodic lancinating, sharp, stabbing, unilateral pain in the distribution of one or more of the trigeminal nerve branches, most often V2
• Unilateral; usually does not affect both sides at the same time
• Although episodes typically last only a few seconds and come in consecutive chains, each total attack may last up to 15–20 min
• Attacks may occur daily or several times per week/month
• Patients usually have no symptoms between attacks (dull pain between attacks suggests persistent compression, e.g., tumor)
• Attacks are often triggered by innocuous stimulus like touching, chewing, facial motion, and cold breezes somewhere in the distribution of the affected nerve
• To minimize attacks, patients often refrain from talking, facial expressive motion, or even eating
• With severe attacks, patients can become easily dehydrated from avoiding beverages and food
Evaluation
• History
• Physical examination is often normal
Loss of corneal reflexes, sensory changes, pain that crosses the midline and bilateral pain at the same time → rule out other causes
• Diagnostic imaging: Head CT or MRI/MRA atypical form
• Patients experience pain constantly or for an unusually prolonged period
• This can be an extremely debilitating condition that reduces one’s quality of life
Treatment
• Pharmacologic therapy
• 80% of patients respond to treatment with carbamazepine (rigid lab monitoring for bone marrow suppression required); improvement sometimes occurs within hours; up to 20% of patients, especially elderly patients, have side effects like ataxia, drowsiness, and confusion; bone marrow depression has also been reported
• Clonazepam and gabapentin with baclofen have also been used
• If immediate relief is required, use IV lidocaine or phenytoin until patient is able to tolerate oral medication
• Second line: Consider opioids
• Lifestyle modification: Especially avoiding triggers (e.g., touching, chewing, facial motion, cold breezes)
• Behavioral modification
• Alternative therapy (e.g., biofeedback, relaxation therapy, acupuncture)
• Interventional therapy
• Trigeminal nerve or Gasserian ganglion block (mostly combination of preservative-free local anesthetic and corticosteroid)
• Neurolysis, percutaneous rhizotomy, gamma knife radiosurgery
• Percutaneous stereotactic radiofrequency thermal rhizotomy
• Stereotactic thalamic stimulation
• Microvascular decompression surgery (e.g., Janetta’s procedure)
Occipital Neuralgia
Pain can be neuropathic, myofascial, or referred, as well as secondary to osteoarthritic changes of the cervical spine.
Etiology
• Occipital nerve entrapment due to occipital artery, scar tissue, mass
• Trauma from cervical or cranial surgery, h/o whiplash injury
• Cervical myofascial pain disorders or dystonias
• Referred pain from cervical discs or facet joints
Symptoms
• Pain in distribution of greater and lesser occipital nerve
• Unilateral constant, dull, achy, with paroxysmal sharp stabbing sensations
• Pain associated with occipital nerve tenderness, myofascial pain, and trigger points in the cervical musculature
• Possible association with trigeminal neuralgia and tension-type, migraine, or autonomic changes of cluster headaches
Evaluation
• History and physical examination
• Tenderness to palpation over base of occipital nerve
• Tinel sign in area innervated by occipital nerve
• Diagnostic imaging: head CT or MRI/MRA to rule out secondary causes
Treatment
• Pharmacologic therapy
• First line: NSAIDs, anticonvulsants, TCAs
• Second line: Opioids
• Lifestyle and behavioral modifications; avoiding triggers
• Alternative therapy: Biofeedback, relaxation therapy, yoga, acupuncture, TENS
• Interventional therapy
• Occipital nerve block with preservative-free local anesthetic +/- corticosteroids
• Upper cervical FMBB with preservative-free local anesthetic/corticosteroids
• Upper cervical FJI with preservative-free local anesthetic +/- corticosteroids
• RFL of upper cervical facet medial branch or occipital nerve after diagnostic block
• Botulinum toxin injection in cervical musculature especially for myofascial pain component
• Occipital nerve neurolysis via alcohol, phenol injection, or cryoablation
• Occipital nerve stimulator
• Surgery
• Occipital nerve decompression surgery
• Occipital neurectomy or C2 ganglionectomy
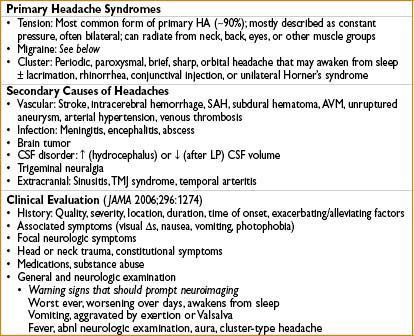
HEADACHE
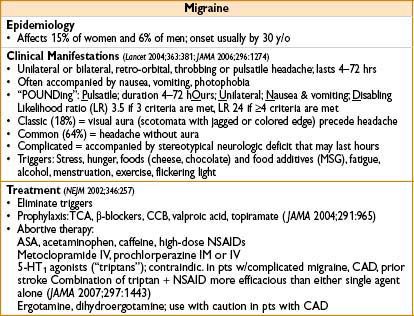
MYOFASCIAL PAIN SYNDROME (MPS)
Epidemiology
• Females > males (54–65% vs. 37–45%); onset usually 20–50 yrs of age
Etiology
• Most common cause of MPS is trauma and acute overload of muscles (e.g., microtrauma from repetitive movement, mechanical stress, muscle overload from poor posturing, leg length discrepancy, prolonged immobility, overuse of unconditioned muscles)
Pathophysiology
• Myofascial trigger points show spontaneous electromyographic (EMG) activity
• Repetitive microtrauma leads to prolonged muscle contraction and local ischemia (as evidenced by decreased adenosine triphosphate [ATP] and creatinine levels, even though lactate and glycogen levels are normal)
• TrPs possibly caused by sympathetically activated intrafusal contractions
Symptoms
• Cervical region > hips > shoulder > lumbar region in females
• Hips > shoulder > cervical region > lumbar region in males
• Most common sites of latent TrPs: Quadratus lumborum 45%, gluteus medius 41%, gluteus minimus 11%, piriformis muscle 5%
• Slight decrease in ROM, easy fatigability, slight loss of dexterity
• Flare-ups can occur from additional injury to the same area
• TrPs may remain latent for years after injury
• Latent TrPs can be activated by intense heat or cold, damp weather
Evaluation
• No widely accepted diagnostic criteria exist
• TrPs are the pathognomonic clinical findings; usually 3–6 mm in size in the middle of the muscle belly, coinciding with the motor point
• Most commonly affected regions: The masseter, splenius capitis, trapezius, levator scapulae, supra- or infraspinatus, quadratus lumborum, gluteus medius, and tensor fascia latae muscle. Impaired ROM in affected area is common
• Spot tenderness (often with hyperalgesia or hyperesthesia) upon palpation
• Jump sign (patient may cry out or jump in response to palpation of TrP)
• Twitch response with transient muscle shortening TrP is actively stimulated
• Knowledge of TrP referral patterns is essential since typically they do not follow dermatomal or peripheral nerve root patterns (e.g., palpating cervical spine TrPs often refers pain to the face and head)
• Muscle biopsy from TrP shows a “moth-eaten” or “waxy degeneration” appearance
Treatment
• Interventional therapy: Trigger point injection (TPI)
• Different needle techniques and substances used for injection
• Follow TPIs with myofascial stretching, physical therapy
• It has been recommended to limit TPIs to once every 3 mos
• Stretch and spray
• Physical therapy (e.g., stretching and strengthening, improvement of poor posture)
• Pharmacologic therapy
• Acetaminophen, NSAIDS, topicals (e.g., local anesthetic or anti-inflammatory patches/ointment/cream, single substance or compound creams)

FIBROMYALGIA
• Generalized soft-tissue pain syndrome with symptoms not only limited to pain
Incidence
• 0.2–2% of the general population between 18–65 yrs
• 2nd most common disorder seen by rheumatologist (after OA)
• Female-to-male ratio is 10:1; 8 times more prevalent in families
Etiology
• Musculoskeletal pain of unknown etiology (possible link to virus that is also responsible for chronic fatigue syndrome)
• No specific risk factors, but associated with autoimmune disease (e.g., SLE, RA, Sjögren’s syndrome, OA, spinal stenosis, hypothyroidism, GH deficiency) and often coincides with stress, trauma, and decreased growth hormone secretion
• Mechanism: Central sensitization resulting in heightened pain sensitivity (blockade of N-methyl-D-aspartate [NMDA] receptors may diminish pain)
• Other associated findings: Abnormal non-rapid eye movement (REM) sleep, diminished hypothalamic-pituitary axis, growth hormone deficiency, central hypodopaminergia, abnormal serotonin metabolism, muscle disuse or deconditioning
• Polymorphisms of genes in serotoninergic, dopaminergic and catecholaminergic systems
Symptoms
• Disease complex entails pain plus three key domains: Mood, sleep, and stress
• Classic symptoms are widespread/diffuse chronic pain patterns and include allodynia or dysesthesia, frequently symmetric distribution, fatigue, weakness, chronic sleep disturbances, functional bowel disturbances, anxiety, depression
• Triggers: Physical and emotional trauma, infection
• Frequently associated symptoms (e.g., fatigue, chronic HA, anxiety, depression, interstitial cystitis, inflammatory bowel syndrome)
Evaluation
• Can be difficult: Essential symptoms still controversial; often diagnosis of exclusion
• History
• Fibromyalgia impact score questionnaire (FIQ), family history, associated symptoms
• Physical examination
• Lower heart rate variability
• Criteria according to the American College of Rheumatology (ACR, 1990)
• Widespread pain lasting >3 mos, affecting all four quadrants of the body; i.e., both sides, and above and below the waist; axial skeletal pain present (cervical spine or anterior chest or thoracic spine and low back)
• Tender points in at least 11 out of 18 locations on digital palpation
• Old criteria focus on TP and do not include fatigue, cognitive symptoms (part of new symptom severity [SS] score)
• Approximately 4 kg/cm2 of force is required to elicit tender point pain (8.8 lb/cm2), which is about the amount required to blanch the thumbnail
• ACR Preliminary Diagnostic Criteria [Arthritis Care & Research 2010;(62)5:600–610]
• Patient satisfies diagnostic criteria for fibromyalgia if 3 conditions are met:
• Widespreadpain index (WPI) ≥7 and symptom severity (SS) scale score ≥5 or WPI 3–6 and SS scale score ≥9
• Symptoms have been present at a similar level for at least 3 mos
• The patient does not have a disorder that would otherwise explain the pain
• WPI number of areas in which patient had pain over the last week (total score between 0 and 19)
• The SS scale score is the sum of the severity of the 3 symptoms (fatigue, waking unrefreshed, cognitive symptoms) plus the extent (severity) of somatic symptoms in general. The final score between 0 and 12.
• Laboratory studies (none are confirmatory or diagnostic)
• CRP, ESR, UA, TSH, HbA1C to determine other disease processes
• Plasma: Decreased levels of cortisol, neuropeptide Y, insulin-like growth factor
• Cerebrospinal fluid: Elevated levels of substance P, endogenous opioids (e.g., endorphins and enkephalins), nerve growth factor, excitatory amino acids; decreased levels of metabolites for serotonin, norepinephrine, and dopamine
• Diagnostic imaging (none are confirmatory or diagnostic)
• Decreased blood flow within the thalamus, basal ganglia, and midbrain
• Differential activation in response to painful stimulation
• Disruption of dopaminergic reactivity in response to a tonic pain stimulus within the basal ganglia
• Reduced availability of opioid receptors in the ventral striatum/nucleus accumbens
Treatment
American Pain Society Guidelines
• Alternative therapy
• Education (fibromyalgia knowledge, nutrition, physical and mental health), aerobic exercise, tai chi, cognitive-behavioral therapy, lifestyle modification (avoidance of caffeine/nicotine/stimulants, exercise, sleep hygiene), relaxation therapy [strong evidence]
• Stretching and strengthening exercise [moderate evidence]
• Acupuncture, trigger point injections [weak evidence]
• Pharmacologic therapy
• First line [strong evidence] anticonvulsants (e.g., pregabalin), tricyclic antidepressants (e.g., amitriptyline), SSRIs (e.g., fluoxetine, sertraline), SNRIs (e.g., milnacipran, duloxetine, venlaxapine), cyclobenzaprine, tizanidine, sodium oxybate
• Second line [moderate evidence] tramadol (other opioids not recommended, unless patient refractory to other therapies, decreased effectiveness of opioids possibly due to increased levels of endogenous opioids and hereby oversaturation of receptors in question), tapentadol
• Third line [weak evidence] acetaminophen, NSAIDs (mostly effective in patients with pain syndromes like arthritis and fibromyalgia)
• For frequent flare-ups: Ketorolac or corticosteroids can be tried
CANCER PAIN
• Somatic, neuropathic, cancer-related bone pain & visceral pain or combination of
• Multiple etiologies: e.g., plexopathies, metastasis, procedure related pain (postmastectomy/post-thoracotomy pain syndrome, phantom pain, radiation-induced neuritis, polyneuropathy from chemotherapy)
Treatment
• Treatment should be symptomatic & combined with oncologic treatments
• Follow the 3-step (plus step 4, interventional procedures) WHO analgesic ladder (Fig. 29-1)
MANAGEMENT OF NEUROPATHIC PAIN
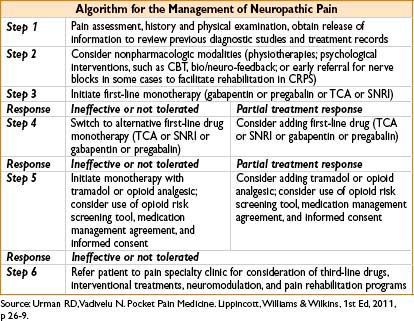
SELECTED INTERVENTIONAL PROCEDURES
Mixture of preservative-free local anesthetic (lidocaine 1–2%, bupivacaine 0.25–0.5%), nonparticulate steroids (40–120 mg), and preservative-free normal saline is used in various combinations for ESI/CSI, SNRBs, FJIs, and FMBBs
• Epidural/caudal/transforaminal steroid injections (ESI/CSI/TFE)
• For treatment of LBP, spinal stenosis, failed back surgery syndrome, radiculopathy
• Interlaminar, transforaminal, or caudal approach
• Peripheral nerve blocks
• For diagnosis or treatment
• e.g., Occipital/trigeminal/facial/lateral femoral cutaneous nerve blocks
• e.g., Brachial plexus block, intercostal block, paravertebral block
• Ilioinguinal/genitofemoral nerve blocks, facet medial branch blocks
• Pain relief for neck & back pain (pain elicited esp. by neck/back extension)
• Blocks medial branch of posterior ramus of spinal nerve
• For severe & prolonged pain, consider denervation of facet joints by RF
Radiofrequency (RF) Neurolysis
• Interrupts nerve conduction for 3–18 mos
• Indications: Denervate facet joints, splanchnic nerve (for abd pain), cranial nerve ganglion (e.g., trigeminal ganglion)
• Conventional RF: High temp. 80°C applied for 60–90 sec at each level
• Only pulsed RF is recommended for peripheral nerves
• Usually done at 42–45°C for 120–180 sec
• Forms a lesion if neural temp. exceeds 45°C
Cryotherapy
• Definition: Extreme cold to freeze & destroy nerves & tissue
• Mechanism: Cell injury by necrosis (resulting from freezing & thawing)
• Technique: Liquid nitrogen most commonly used (argon & N2O possible), desired temp. –50 to –60°C; usually one or two 5 to 30 sec freeze–thaw cycles performed q4–6wk
• Stellate ganglion block
• Definition: Cervical/thoracic sympathetic ganglion block (fusion of inferior cervical & 1st thoracic ganglion)
• Indications: CRPS (I & II), phantom pain, refractory angina, vascular insufficiency (Raynaud’s syndrome), frostbite, scleroderma
• Side effects: Horner’s syndrome, warm extremity


• Provocative Discography
• Performed to determine if back pain is discogenic (arising from disc)
• Involves injection of contrast media into disc in question (pain generator) plus at least 1-2 levels above or below as control level
• Test = positive when concordant pain is produced
• Treatment of discogenic pain
• Initial therapy = dynamic lumbar stabilization exercises
• If pain persists → percutaneous intradiscal RF ablation or spinal fusion
• Spinal cord stimulation (SCS)
• Indications: CRPS, periph vasc dz, failed back surgery syndrome, & angina (occasionally phantom pain, PHN, spinal cord injury)
• Mechanism: Rectangular pulses delivered to epidural space via implanted electrodes (connected to pulse generator)
• Efficacy: 50% pain relief in about 50% of people, especially beneficial for extremity pain
• Intrathecal pumps: Deliver agents into intrathecal space (e.g., commonly used, mostly in combination are opioids [morphine], local anesthetics, clonidine, baclofen)
• Tunneled catheters (e.g., provides neuraxial analgesia)
(intrathecal/epidural) frequently used for the treatment of advanced cancer pain
• Neurolytic procedures
• Performed to relieve visceral pain often in cancer pts with short life expectancy
• Blocks include: Celiac plexus block, superior/inferior hypogastric plexus blocks & neurolysis of ganglion of impar (sympathetic ganglion in sacrococcygeal junction)
• Techniques: Injections of alcohol or phenol; cryotherapy; RF lesioning

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








