157 Over-the-Counter Medications
• Acetaminophen poisoning should be considered in patients presenting with over-the-counter medication misuse or overdose.
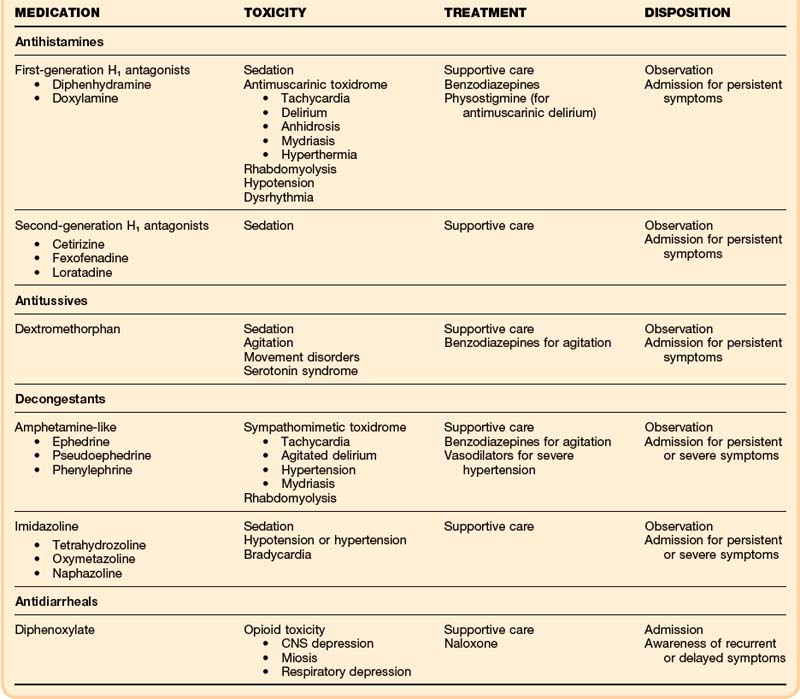
• Poisonings with antihistamine medications may manifest with antimuscarinic toxicity and sedation, but cardiac toxicity and seizures are also possible.
• Treatment for antihistamine poisoning is supportive care, but sodium bicarbonate and physostigmine may be helpful adjuncts.
• Dextromethorphan poisoning manifests as sedation, movement disturbances, and psychoactive dysphoria. Most of these effects are mediated by N-methyl-D-aspartate, rather than by opioid receptor activity.
• In overdose, poisoning with oral amphetamine-like decongestants may manifest as a sympathomimetic toxidrome.
• Imidazoline ocular and nasal decongestants such as oxymetazoline (Afrin) tetrahydrozoline (Visine), and naphazoline (Naphcon) may cause significant sedation when they are ingested orally.
• Diphenoxylate (Lomotil) can cause recurrent and delayed respiratory depression.
• Dietary supplements are mostly safe but are unregulated. Common conditions leading to poisoning include mislabeling, variations in concentration, contamination with unintended agents, and intentional adulteration.
• Vitamin A toxicity may cause increased intracranial pressure with associated symptoms.
Antihistamines
Epidemiology
Antihistamines are among the most frequently used medications in the United States.1 Most of these drugs are available without a prescription. Although their efficacy is questionable, antihistamines are widely used for the symptomatic relief of cold and allergy symptoms. They are also found in nonprescription sleeping aids. Because of widespread access, these agents are commonly ingested intentionally, in suicide attempts, and unintentionally, particularly by children. Approximately 90,000 cases of antihistamine ingestions are reported to poison centers in the United States every year, and almost half of those involve children younger than 6 years.2
Presenting Signs and Symptoms
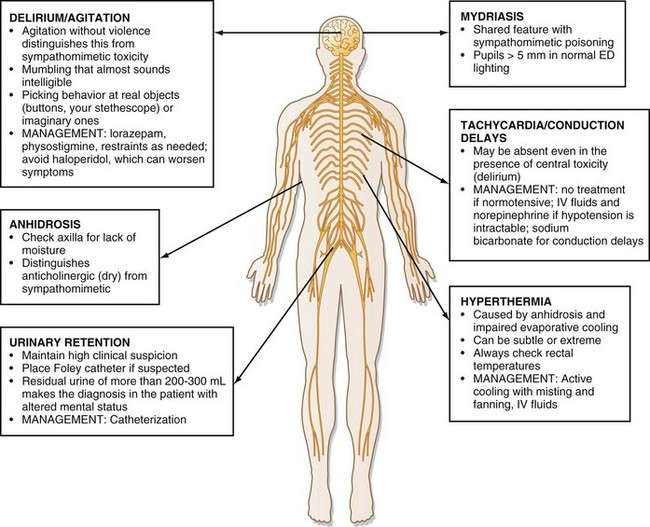
Although sedation is common with larger ingestions, signs of antimuscarinic toxicity may also be present (Box 157.1). In addition, patients may have mild hypotension or more worrisome wide complex dysrhythmia resulting from sodium channel–blocking effects of some antihistamines (e.g., diphenhydramine). Cardiovascular toxicity associated with some antihistamines is indistinguishable from that associated with cyclic antidepressants (Fig. 157.1).
Differential Diagnosis and Medical Decision Making
The differential diagnosis of antihistamine toxicity is broad because many medications, street drugs, and disease processes can cause a presentation characterized by sedation or delirium, or both (Box 157.2). The emergency physician must consider nontoxicologic causes of altered mental status, such as infection and traumatic brain injury, and evaluate for these nontoxicologic conditions accordingly if the presence of the conditions cannot be excluded by other means. Computed tomography of the head and lumbar puncture may be warranted. In particular, patients presenting with antimuscarinic toxicity may be delirious, hyperthermic, and tachycardic, features that mimic infectious causes.
Treatment
Hospital treatment should also be focused on supportive care with assessment of airway, breathing, and circulation. Particular attention should be paid to controlling agitation and hydration (Table 157.1). Agitation should be treated with benzodiazepines in doses titrated to desired effect (e.g., lorazepam, 1 to 2 mg by intravenous [IV] push to effect). In addition to chemical restraint, physical restraint for patient and staff safety may be needed. Hydration should be addressed with 1- to 2-L boluses of 0.9% saline solution to ensure adequate urine output.
In patients who present within 1 hour of drug ingestion and who are alert and cooperative, activated charcoal (50 g or 1g/kg up to 50 g in children) should be considered. Data in humans are insufficient to support the use of activated charcoal beyond 1 hour.3
Physostigmine is a reversible acetylcholinesterase inhibitor that crosses the blood-brain barrier; it increases synaptic acetylcholine and may temporarily reverse antimuscarinic delirium. Peripheral signs may also be reversed. It may also be used therapeutically to control agitation.4 Physostigmine may have more value as a diagnostic tool by precluding the need for invasive tests (e.g., lumbar puncture) if complete reversal of delirium is achieved following administration.5 Beyond diagnostic use, the role of physostigmine in the treatment of most antimuscarinic poisonings with minor symptoms is debatable, and caution should be used if a possibility of tricyclic antidepressant ingestion exists.
Antitussives: Dextromethorphan
Epidemiology
Dextromethorphan was approved by the U.S. Food and Drug Administration (FDA) as an over-the-counter antitussive in 1958. It is a common component of cold preparations, and its nonmedical use (abuse) appears to be increasing, especially among adolescents.6 Abuse of Coricidin (known on the streets as “Skittles”) and Robitussin (known as “DXM” and “robo”) has highlighted dextromethorphan’s abusive potential.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree