Chapter 120 Organ System Considerations that Affect Anesthetic Management
Cardiovascular Performance
Changes with Development
Cardiac assessment of the PICU patient requires a knowledge of normal cardiovascular growth and development and an understanding of the influence of disease and the effects of anesthesia on cardiovascular function. The myocardium of the neonate is less compliant than that of the adult and the neonate’s cardiac output depends primarily on the heart rate. Cardiac output increases substantially with age, whereas cardiac index (cardiac output divided by body surface area) ranges from 2.5 to 4.2 L/min/m2 throughout life. Sympathetic innervation of the neonatal heart is not fully developed, and myocardial catecholamine stores are limited.1 The response of the neonatal heart to vasoactive drugs is attenuated and the capacity for peripheral vasoconstriction during hypovolemia is reduced, probably because of the immature baroreceptor and α-adrenergic receptor systems.2 Systemic vascular resistance is normally 800 to 1200 dynes • sec/cm–5 at birth and reaches the adult value of 1600 by 1 to 2 years of age. Systolic and diastolic blood pressures increase nonlinearly with age.
Effects of Disease
Disease states can affect cardiovascular performance by their effects on the pulmonary and peripheral vasculatures and by their direct effects on the myocardium. Changes in myocardial compliance and filling pressures can profoundly influence myocardial performance. In addition, factors associated with disease states such as hypoxia, hypercarbia, acidosis, and hypothermia can affect pulmonary and systemic vascular resistances and thereby further modify myocardial function. The effects of changes in preload (i.e., the effects of fluid challenges) on the patient’s blood pressure, cardiac output, and stroke volume and the clinical effects of changes in afterload on heart rate and myocardial contractility all influence the anesthesiologist’s care of critically ill children.
Effects of Anesthetic Agents
Anesthetic agents affect myocardial performance. During induction of anesthesia, potent inhaled agents such as halothane and isoflurane are associated with higher incidences of bradycardia, hypotension, and cardiac arrest in infants and children than in adults.3 These cardiovascular depressant effects appear to be more pronounced in infants than in older children. Diaz and Lockard4 found that more than 70% of healthy newborns had greater than 30% decrease in systolic blood pressure during induction with halothane, whereas Friesen and Lichtor3 noted that infants 1 to 6 months of age had a 40% decrease in mean atrial pressure and a 30% decrease in heart rate when they received a halothane and nitrous oxide anesthetic. In a study of healthy children, 1.5 to 12 years of age, undergoing halothane anesthesia, Barash et al.5 noted that systolic blood pressure and heart rate decreased in a dose-dependent manner. Similar hemodynamic effects are noted in infants anesthetized with isoflurane.6
Because it can be problematic to insert invasive monitors in unsedated, awake children, much of the information about the potent inhaled anesthetic agents and their effects on the determinants of cardiac output is derived from animal studies. In a neonatal piglet model, Boudreaux et al.7 noted that the major adverse effect of halothane was its negative inotropic effect and not its negative chronotropic or unloading activity. In similarly designed studies, Schieber et al.8 observed that, although isoflurane reduced contractility and decreased blood pressure and systemic vascular resistance more than did equipotent concentrations of halothane, cardiac index was better preserved in the isoflurane-anesthetized animals. Thus when compared with halothane, the direct myocardial depressant effect of isoflurane is offset by its effect on the peripheral vasculature, which results in afterload reduction.
Desflurane and sevoflurane are two new potent inhalational anesthetic agents. Because of their low blood solubility, they afford patients rapid induction and rapid awakening.9–14 Desflurane has a blood gas solubility coefficient (0.42) that is similar to nitrous oxide in children. However, desflurane’s pungent airway properties result in a high incidence of laryngospasm, coughing, and hypoxemia that limit its utility as an induction agent in nonintubated children.14 The cardiovascular profile of desflurane is age dependent.12 Compared with awake values, arterial blood pressure decreased in children anesthetized with 1 minimal alveolar concentration (MAC) of desflurane by approximately 30%, whereas the heart rate decreased or remained the same. Thus at 1 MAC, desflurane, like isoflurane and halothane, appears to attenuate the baroresponse in children. Weiskopf et al.15 demonstrated that rapid increases in desflurane from 0.55 to 1.66 MAC in adults can transiently increase arterial blood pressure and heart rate. This cardiovascular excitation is associated with an increase in sympathetic and renin-angiotensin system activity.
Information on the hemodynamics of sevoflurane in children suggests that sevoflurane (blood gas coefficient of 0.68) appears to produce the same hemodynamic effects as isoflurane.10 In adults anesthetized with sevoflurane or isoflurane, administration of exogenous epinephrine had similar dysrhythmogenic properties.16 In a study of children, using echocardiograms, in which sevoflurane and halothane were compared at equal MAC, Holzman et al.17 noted sevoflurane had fewer myocardial depressant effects than sevoflurane.
The synthetic opioids may offer more hemodynamic stability than the inhaled anesthetic agents.18–20 Robinson and Gregory21 were the first to report the safety and efficacy of high-dose fentanyl anesthesia in children, in a study of premature infants undergoing patent ductus arteriosus ligation. In subsequent reports on pediatric patients, Hickey and Hansen22 documented the safety of opioid anesthesia in children with complex congenital heart disease. Although these investigators noted that high doses of fentanyl (50 and 75 μg/kg) decreased heart rate and mean arterial pressure (MAP) by 7% and 9%, respectively, in patients with bidirectional shunts, opioids had a salutary effect on pulmonary vascular resistance, increasing transcutaneous oxygenation by 45%.
Because of the prolonged respiratory and sedative effect associated with moderate- and high-dose administration of fentanyl and its congeners, shorter-acting opioids may offer the advantage of more predictable control with a similar cardiovascular profile than the longer-acting opioids. Remifentanil, a new synthetic opioid agonist with an ultrashort half-life, has been introduced into clinical practice. Remifentanil is metabolized by plasma and tissue esterases. It is independent of organ elimination. Consequently, its kinetic parameters do not change with the duration of infusion. This flat, context-sensitive half-time coupled with its ultrashort half-life (7 to 10 minutes) allow better drug effect predictability. Pharmacokinetic studies in children demonstrate faster clearances and larger volumes of distribution in neonates compared with older infants and children. In vitro studies by Olgatree et al.23 have demonstrated that remifentanil has no significant direct negative inotropic effects on the myocardium and that β-adrenergic stimulation of the heart remains intact. Comparative studies of remifentanil to inhaled anesthetic agents in children undergoing pyloromyotomy surgery suggest that the short half-life of remifentanil may be beneficial with regard to postoperative respiratory changes.24,25 Because of remifentanil’s ultrashort half-life and its nonaccumulation with prolonged infusion, it may be a beneficial agent for the short-term (less than 12 hours) sedation of infants and children in the PICU setting. However, the rapid development of tolerance and cost issues will likely preclude its use for longer periods of time.
Dexmedetomidine, a sedative analgesic that has become increasingly popular, like clonidine is an α2-adrenergic agonist and is highly selective with a α2/α1 ratio of 1600:1. Stimulation of these receptors in the central nervous system (CNS) and the spinal cord produces sedation, anxiolysis, analgesia, deceased MAC of inhalational anesthetic agents (increased sensitivity), decreased renin and vasopressin levels with increased diuresis, decreased sympathetic tone with decreased heart rate and blood pressure.26 Dexmedetomidine has a rapid distribution phase (6 minutes) and an elimination half life of 2 hours.27 Petroz et al.28 demonstrated that children, 2 to 12 years of age, have similar pharmacokinetics to adults. Rodarte et al.29 studied the pharmacokinetics in infants 1 to 24 months of age. He concluded that infants have a faster clearance of dexmedetomidine than adults (27 mL/kg/min versus 13 mL/kg/min). A loading dose of 1 μg/kg and continuous infusions of 0.2 to 0.7 μg/kg/hr have been used in various clinical scenarios to produce sedation-analgesia. The most common adverse events are hypotension and bradycardia, whichare enhanced in the presence of cardiac comorbidities or when dexmedetomidine is used with other medications that have negative chronotropic effects (propofol, succinylcholine, digoxin, pyridostigmine).30–32 When a loading dose of dexmedetomidine is administered, there is a biphasic response. The initial response is an increase in blood pressure with a decrease in heart rate, followed by a decrease in blood pressure and a further decrease in heart rate.33 This biphasic effect is thought to be due to dexmedetomidine’s initial ability to stimulate peripheral postsynaptic α2b-adrenergic receptors resulting in vasoconstriction, followed by the more intense central CNS effects on α2a-adrenergic receptors causing sympatholysis. Bloor et al.33 administered boluses of 0.25, 0.5, 1.0, and 2.0 μg/kg to healthy volunteers and noted a decrease in mean arterial blood pressure (MAP) respectively of 14%, 16%, 23%, and 27%. Cardiac output decreased 20% following a loading dose of 1 μg/kg in the first minute and returned to 90% of baseline after 60 minutes. When a loading dose of 2 μg/kg was administered, cardiac output decreased by 60% and returned to 85% of baseline after 1 hour. Venn et al.34 studied the effects of dexmedetomidine in 66 patients with comorbidities who were in the ICU, mechanically ventilated, and who received a loading dose of 1 μg/kg followed by a continuous infusion of 0.2 to 0.7 μg/kg/hr. Hypotension and bradycardia (≥30% decrease from baseline) was observed in 18 of the 66 patients. Reports of bradycardia and sinus arrest have also been noted by Ingersoll-Weng.31 Khan et al.35 reported similar effects of dexmedetomidine during anesthesia with isoflurane. The majority of the effects occurred with end-tidal isoflurane levels greater than or equal to 1%. Animal studies and studies on isolated human papillary muscle have demonstrated no direct negative inotropic effects on myocardial contractility.36 The sympatholytic effects of dexmedetomidine on pediatric patients have been evidenced by Muktar et al.37 Thirty infants and children undergoing CPB were randomized to receive dexmedetomidine (1 μg/kg load followed by a continuous infusion of 0.5 μg/kg/hr) or placebo. Plasma cortisol norepinephrine, epinephrine, and glucose levels were significantly less in the dexmedetomidine group.
Propofol is a sedative hypnotic that is widely used as an induction agent in anesthesia. It is also used as a continuous infusion for prolonged sedation in the ICU. Its popularity in the ICU setting comes from its rapid clearance that allows quick awakening for rapid weaning or neurologic evaluation. Propofol is suspended in a lipid emulsion, which when administered by a continuous infusion results in a significant lipid load. Induction doses for anesthesia vary from 2 to 3 mg/kg, while ICU sedation doses vary from 50 to 250 μg/kg/min. Induction doses of propofol can cause a 10% to 15% decrease of MAP as well as bradycardia, especially when coadministered with other vagotonic drugs. Propofol has a modest negative inotropic effect, due to antagonism of β-adrenergic receptors and calcium channels.38 Since 1992, there have been increasing reports of a fatal adverse reaction that has been termed the Propofol Infusion Syndrome (PRIS).39 To date, 61 cases (32 pediatric and 29 adult) have been reported, and there have been 20 pediatric and 18 adult deaths.40 PRIS is characterized by severe intractable bradycardia that leads to cardiac failure, severe metabolic acidosis, hyperlipidemia, rhabdomyolysis with consequent hyperkalemia, and renal failure.41 Prolonged propofol infusions (more than 48 hours) and infusion rates greater than 4 mg/kg/hr have been linked to PRIS. Priming factors such as critical illness (respiratory failure and traumatic brain injury) and triggering factors such as catecholamine and steroid infusion are associated with the syndrome.44 The underlying mechanism of PRIS is not well understood. It is speculated that high doses of propofol inhibit the mitochondrial respiratory chain. As a result, there is a decreased ATP production and a decreased mitochondrial lipid metabolism, with an accumulation of toxic long fatty acid chains that are arrhythmogenic.45–47 Management of PRIS is very difficult and consists of prompt recognition and interruption of the infusion. Aggressive cardiac resuscitation must be initiated early on with high dose inotropes, fluid administration, and the use of pacing devices. The early administration of hemodialysis and hemofiltration together with ECMO has improved survival.48–50
Anemia and Transfusion
Concerns about transfusion-related disease transmission have forced clinicians to reassess transfusion criteria. Rothstein51 previously recommended that in patients younger than 3 months of age, hemoglobin concentration should be greater than 10 g/dL, whereas in children older than 3 months, a hemoglobin concentration of 9 g/dL was adequate. Slogoff52 concluded that in normovolemic adults, a hematocrit of 20% (hemoglobin 7 g/dL) is adequate. Carson et al.53 retrospectively reviewed mortality in 125 adult surgical patients who refused blood transfusion for religious reasons. Mortality correlated inversely with preoperative hemoglobin level and directly with operative blood loss. No operative deaths occurred among patients with a preoperative hemoglobin level above 8 g/dl and an operative blood loss of less than 500 mL.
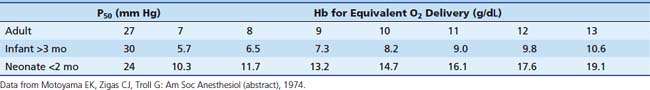
Minimum acceptable hemoglobin (and approximate hematocrit) levels can be inferred from anticipated tissue oxygen delivery (Table 120-1). A hemoglobin concentration of 7 to 8 g/dL (hematocrit 21% to 24%) is reasonable for adults and children based on the previous discussion. Minimum values chosen for infants should be more conservative (i.e., ensure greater oxygen delivery to tissues) than those for older patients. This takes into consideration the higher oxygen consumption of infants and provides them with a larger margin of safety against hypoxic injury. Infants younger than 2 months probably require a hemoglobin level of 13 g/dL (hematocrit of 40%); infants older than 3 months require a hemoglobin level of 7 g/dL (hematocrit of 21%). Infants aged 2 to 3 months are in a transitional phase, and minimum hemoglobin levels are more difficult to predict. These values are intended as guidelines only; each patient must be considered individually.
Patients with chronic anemia increase oxygen delivery by increasing cardiac output. Although the potent inhaled anesthetic agents decrease myocardial function and cardiac output and thereby decrease oxygen delivery, these side effects are offset by the decrease in oxygen consumption that occurs in anesthetized patients. The need to transfuse blood in the perioperative period frequently is determined by the patient’s underlying hemodynamic stability, the type of surgery anticipated, and the known risks of administering blood products. In general, unless the procedure is minor, blood should be available for all ICU patients. Because ICU patients frequently are monitored with invasive catheters, serial measurements of hematocrit or hemoglobin usually can be obtained during the operative procedure to determine whether transfusion is warranted.
Respiratory Failure
When respiratory failure is present, the anesthesiologist must be aware of its precipitating factors. Acute respiratory distress syndrome (ARDS) is a clinical syndrome of respiratory distress, poor pulmonary compliance, and hypoxemia that usually occurs after a nonpulmonary condition such as shock, trauma, sepsis, or an unexplained condition leading to pulmonary parenchymal disease and respiratory failure. Direct mortality from respiratory failure is approximately 10% to 20%; overall mortality may be as high as 65%. ARDS was first described as a clinical entity occurring in pediatric patients in 1980.54 Recognized factors predisposing pediatric patients to the development of ARDS include severe infection, cardiac arrest, shock, and aspiration. Although the cause of this condition is uncertain, the fundamental defect in the lung is injury to the pulmonary capillary endothelial cells, leading to interstitial and, ultimately, alveolar pulmonary edema. This pulmonary vascular leakage results in hypoxemia, decreased pulmonary compliance, increased pulmonary vascular resistance, and venoarterial shunting.
The mainstays of treatment are endotracheal intubation, continuous positive-pressure ventilation with positive end-expiratory pressure (PEEP), and supplemental oxygen. Invasive monitoring by arterial, central venous, and pulmonary arterial cannulation may be necessary to optimize preload and cardiac output in the presence of high PEEP and high transpulmonary pressures. The patient’s requirements for oxygen, PEEP, and pulmonary toilet must be understood preoperatively. Patients with poor pulmonary compliance challenge the ability to maintain adequate intraoperative ventilation. Preoperative knowledge of the interaction of PEEP with the adequacy of ventilation and cardiovascular function is an important concern of the anesthesiologist. Anesthetic agents can modify not only cardiovascular function, but also respiratory function, by their effect on hypoxic pulmonary vasoconstriction. Patients with ARDS may rely in part on regional hypoxic pulmonary vasoconstriction to minimize intrapulmonary shunting. Shifting of pulmonary blood flow away from atelectatic lung regions has been well described. The administration of vasoactive drugs and potent inhaled anesthetic agents inhibits hypoxic pulmonary vasoconstriction and therefore may exacerbate hypoxemia by increasing intrapulmonary shunting.55,56
Neurologic Injury
The leading form of pediatric trauma is traumatic brain injury (TBI), which accounts for 36% of traumatic deaths in the USA.57,58 Eighty percent of trauma patients have central nervous system injury, and in 60% of these patients, the CNS injury is the most severe. It is estimated that 185 of every 100,000 children experience head trauma requiring hospitalization.58,59 Poor outcomes have been associated with early hypotension, low Glasgow Coma Scale score, altered cerebral blood flow (CBF), hyperglycemia, and deranged autoregulation.59 Neurologic injury may be the primary insult resulting from trauma. However, secondary cerebral injury may result from the development of intracranial hypertension and resultant cerebral ischemia. Recognition and control of elevated intracranial pressure (ICP) are key elements to preventing neurologic deterioration and patient morbidity. Secondary traumatic brain injury can be characterized by an array of biochemical, cellular, and molecular events that are associated with ischemia, excitotoxicity, energy failure, and cell death cascades, all of which result in cerebral swelling, axonal injury, inflammation, and regeneration.60
Intracranial Pressure
The signs of intracranial hypertension in infants and children are listed in Table 120-2. The presence of these signs and symptoms, in conjunction with the baseline findings on neurologic examination, may dramatically alter the patient’s anesthetic care. The anesthesiologist must be aware of ongoing interventions to control ICP. Control of cerebral perfusion pressure is essential for maintaining neurologic function in pathologic states. Cerebral perfusion pressure is expressed as the difference between MAP and the highest value obtained out of the following: central venous pressure, intrathoracic pressure, or ICP. Thus in the presence of intracranial hypertension (ICP ≥15 to 20 mm Hg), higher systemic blood pressures must be achieved to prevent cerebral ischemia. Intracranial compliance depends on the volumes of the intracranial contents, cerebral tissue, blood, and extracellular fluid. The most dynamic of these is the blood compartment. Changes in cerebral blood volume are mediated primarily through changes in cerebral vascular resistance. Cerebral vascular resistance is affected by both intracranial and extracerebral factors.
Table 120–2 Signs of Intracranial Hypertension in Infants and Children
| Infants | Children | Infants and Children |
|---|---|---|
| Irritability | Headache | Decreased consciousness |
| Full fontanelle | Diplopia | Cranial nerve (III and VI) palsies |
| Widely separated cranial sutures | Papilledema | Loss of upward gaze (setting sun sign) |
| Cranial enlargement | Vomiting | Signs of herniation, Cushing triad, pupillary changes |
Regulation of Cerebral Blood Flow
The normal brain receives 15% of cardiac output. Kennedy and Sokoloff61 determined that healthy children have a high CBF (100 mL/100 gm/min), this value decreases to adult values of 50 mL/100 gm/min during the teenage years. Cerebral blood flow regulation depends primarily on the local chemical and metabolic milieu, particularly the concentrations of hydrogen ion, adenosine, and prostanoids. Production of these compounds correlates with normal cerebral activity and metabolic rate. Neurogenic and myogenic components also have minor roles in regulating cerebral vascular resistance.
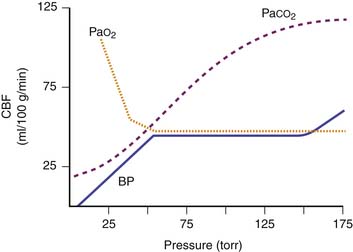
Extracerebral factors that can change cerebral vascular resistance and CBF include the arterial partial pressures of carbon dioxide (PaCO2) and oxygen (PaO2), MAP, and various drugs (Figure 120-1). Cerebral circulation is very sensitive to variations of PaCO2, and has been studied using transcranial Doppler ultrasound.62 CBF varies linearly by 2% to 4% for every variation of 1 mm Hg of PaCO2. Data from anesthetized children show that CO2 vasoreactivity is higher than in adults (13.8% vs. 10.3% change).63 Low blood pressure decreases cerebral vascular vasoreactivity.63 As PaCO2 is rapidly lowered toward 20 mm Hg, there is marked cerebral vasoconstriction and reduction in both CBF and ICP. Theoretically, an acute decrease in PaCO2 below 20 mm Hg may be detrimental by reducing CBF enough to cause cerebral ischemia. The salutary effects of acute hyperventilation on ICP are diminished over time because acute changes in cerebrospinal fluid pH are normalized in approximately 6 hours. Arterial oxygenation within the normal clinical range has little effect on cerebral vascular resistance. However, PaO2 less than 50 mm Hg results in cerebral vasodilation and increased CBF. Hyperoxia in excess of 300 mm Hg may produce cerebral vasoconstriction.
Cerebral autoregulation is a homeostatic process by which the brain maintains a constant CBF over a MAP range from 60 to 150 mm Hg in adults. At an MAP less than 60 mm Hg, symptoms of cerebral ischemia may appear. If the upper limit of MAP for autoregulation is exceeded, the resultant increase in CBF may cause cerebral edema. The autoregulatory curve may shift in the presence of chronic hypertension, intracranial tumors, head trauma, or shock states. This renders the brain more susceptible to ischemic effects.63 The range of MAP over which autoregulation of CBF occurs in infants and children likely shifts in tandem with age-related changes in normal systemic blood pressures and cerebral perfusion pressures. Raju et al.64 suggest that an infant’s mean cerebral perfusion pressure is approximately equal to its gestational age in weeks and that this estimate holds true for growing preterm infants up to 5 weeks after birth.
Effects of Anesthetics on Cerebral Blood Flow
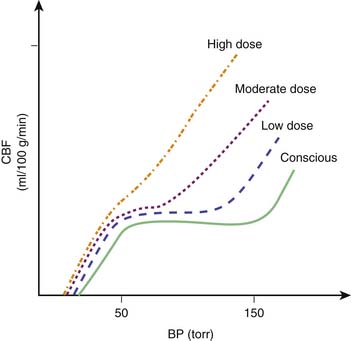
In general, the potent inhaled anesthetic agents impair autoregulation and may cause hypotension and increased CBF (Figure 120-2). Consequently, they must be used cautiously, if at all, in patients with evidence of traumatic brain injury. Halothane and enflurane at 1 MAC abolish cerebral autoregulation. Isoflurane impairs autoregulation less than halothane.65 The effects of anesthetic agents on CBF and cerebral metabolism are summarized in Figure 120-3. In general, the perfect agent would decrease both CBF and cerebral metabolic rate of oxygen (CMRO2). The potent inhaled agents (halothane, enflurane, and isoflurane) “uncouple” the normal relationship between CBF and metabolism and cause marked cerebral vasodilation. Isoflurane is the only inhaled anesthetic agent with which CBF actually may decrease when concomitant hyperventilation to PaCO2 of 20 to 25 mm Hg is used.66 Whereas nitrous oxide alone is known to cause mild cerebral vasodilation and to increase CMRO2 (probably related to inadequate anesthetic depth), these effects are easily countered by the addition of intravenous sedatives, hypnotics, and narcotics. There are limited data regarding the effects of the two newest inhaled agents, sevoflurane and desflurane, on intracranial dynamics. Although the effects of desflurane on CBF have been studied, there is relatively little information regarding the effects of sevoflurane on CNS physiology in human subjects. Artru et al.67 noted that desflurane increases CSF pressure in animals. Ornstein et al.68 showed that in patients with intracranial mass lesions, desflurane and isoflurane have similar effects on CBF. The electroencephalographic effects of desflurane are similar to those of isoflurane, with prominent burst suppression occurring at 1.24 MAC.69
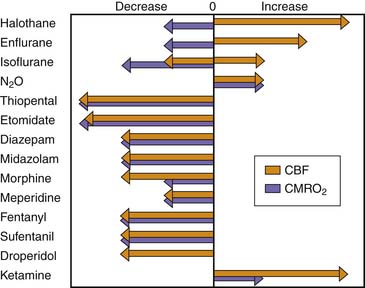
Figure 120–3 Effects of anesthetic agents on cerebral blood flow (CBF) and cerebral metabolic rate of oxygen (CMRO2).
(Modified from Cucchiara RF, Block S, Steinkeler JA: The effects of anesthetic agents on cerebral blood flow and cerebral metabolism: anesthesia for intracranial procedures. In Barash PG, Cullen BF, Stoelters SK, editors: Clinical anesthesia, Philadelphia, 1989, JB Lippincott.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree