184 Opioids
 Pharmacology and Receptor Physiology
Pharmacology and Receptor Physiology
Opioids act as agonists at opioid receptors at presynaptic and postsynaptic sites in various regions of the brain and spinal cord including the periaqueductal gray area of the brainstem, amygdala, corpus striatum, thalamus, and medulla, as well as the substantia gelatinosa (dorsal/posterior horn) in the spinal cord. Opioid receptors are also found in peripheral tissues at afferent pain neurons, in the smooth muscle of the gastrointestinal (GI) tract, and intraarticularly. Agonism at opioid receptors decreases neurotransmission through pain neurons, both in the periphery and in the spinal cord. Opioid receptor agonism also diminishes the brain’s perception of pain. This reduction in nerve transmission occurs through alteration of the release of neurotransmitters such as acetylcholine, norepinephrine, dopamine, serotonin (5-hydroxytryptamine [5-HT]), glutamate, and substance P. Decreased neurotransmission is thought to be secondary to membrane hyperpolarization or decreased release of neurotransmitters from presynaptic vesicles or both.1
Three major subtypes of opioid receptors have been identified: mu, delta, and kappa. All these are G protein–coupled receptors and have seven transmembrane helices with significant sequence homology. Opioid receptor agonists and antagonists interact with one or more of these receptors with varying affinities.1,2 This Greek-derived nomenclature is commonly used by most of the scientific community. In 1996, the International Union of Pharmacology (IUPHAR) recommended a new nomenclature for opioid receptors, having as a goal consistency in naming with other neurotransmitter systems (Table 184-1).3 The traditional Greek notations are used in this text. Several other new receptor subtypes have been identified. Their clinical significance and classification are unclear at this time.
 Pharmacokinetics
Pharmacokinetics
Distribution
Tissue uptake is variable and depends largely on the drug’s lipophilicity. Highly lipophilic compounds such as fentanyl readily penetrate the central nervous system (CNS), the dura of the spinal column, and tissue “reservoirs.” Opioids exhibit varying degrees of plasma protein binding and typically have large volumes of distribution. Serum concentrations of opioids should not be used as a gauge of clinical effect, because fat, skeletal muscle, lungs, and viscera act as reservoirs after opioid administration. Redistribution from saturated tissue depots can produce persistent or recurrent sedation after discontinuation of prolonged infusions of certain opioids such as fentanyl.4
 Clinically Important Effects in the Intensive Care Unit
Clinically Important Effects in the Intensive Care Unit
Analgesia, euphoria, sedation, miosis, and respiratory depression are considered to be the classic opioid effects. In addition, opioids have many more clinically relevant effects, many of which are not typically relevant in the intensive care unit (ICU) setting; these are summarized by physiologic system in Table 184-2.
TABLE 184-2 Summary of Clinical Effects of Opioids by Physiologic System
| System | Clinical Effect |
|---|---|
| Cardiovascular | Hypotension (vasomotor centers and histamine), bradycardia (first or second degree), dysrhythmias (overdose, propoxyphene), QRS prolongation (propoxyphene), QT prolongation (methadone) |
| Dermatologic | Urticaria, flushing, pruritus (centrally mediated) |
| Endocrinologic | Reduced release of antidiuretic hormone (controversial), reduced release of gonadotropin |
| Gastrointestinal | Nausea, vomiting (5-HT2 mediated), delayed gastric emptying, constipation, increased smooth muscle tone (biliary tract, intestinal, pylorus, anal sphincter) |
| Genitourinary | Urinary retention, ureteral spasm, decreased renal function and renal blood flow, antidiuresis, priapism (neuraxial use) |
| Immunologic | Mast cell degranulation/histamine release, cytokine stimulation (IL-1), but true allergic reaction is rare |
| Maternal/fetal | Placental transmission, neonatal blood-brain barrier immature, neonatal respiratory depression and opioid dependence, neonatal withdrawal (seizures) |
| Musculoskeletal | Truncal/chest wall rigidity and myoclonus (fentanyl derivatives) |
| Neurologic | Analgesia, euphoria, sedation, psychotomimesis, seizures (meperidine, propoxyphene, tramadol, rarely fentanyl) |
| Ophthalmic | Miosis, normal or dilated pupils (meperidine, pentazocine, diphenoxylate, propoxyphene, severe systemic hypoxia) |
| Pulmonary | Respiratory depression, antitussive effect, bronchospasm, pulmonary edema |
5-HT, serotonin; IL, interleukin.
Analgesia
Opioids are modulators of pain perception both at the level of the CNS and in the periphery. High concentrations of opioid receptors (largely of the mu subtype) are found in areas of the brain that are associated with analgesia. Cortical effects include decreased reception of painful sensory inputs and enhanced inhibitory outflow from the brain to the sensory nuclei of the spinal cord (dorsal root nuclei). In addition, there is decreased neurotransmission from peripheral afferent pain neurons to the spinal cord and from the spinothalamic tract to the brain. The net effect is decreased perception of nociceptive information. Analgesia is mediated by the mu, delta, and kappa opioid receptor subtypes (see Table 184-1). Morphine also appears to be an effective analgesic (via the mu opioid receptor) when administered intraarticularly.5 Tolerance develops to the analgesic effects with repeated use.
Very low doses of naloxone (e.g., 0.25 µg/kg/h) improve the efficacy of morphine analgesia, whereas at higher doses (1 µg/kg/h), analgesia is obliterated by naloxone. The mechanism of this effect is unclear.6
Euphoria
The euphoric effects of opioids are typically described as pleasant, floating sensations accompanied by a decrease in anxiety and distress. Not all exogenous opioids induce the same degree of euphoria. Activation of the mu/delta receptor complex in the ventral tegmental area, followed by dopamine release in the mesolimbic system, is most likely responsible for these effects.7
The degree of lipophilicity and CNS penetration is directly proportional to the euphoric properties of the opioid. For example, heroin, which enters the CNS with relative ease, is associated with greater euphoria than is the less lipophilic opioid, morphine.8 Fentanyl produces euphoric effects akin to those of heroin and is occasionally used as an adulterant in illicitly obtained heroin.9 The apparently enhanced euphoric effect of meperidine may be related to its lipophilicity and its ability to alter serotonergic neurotransmission.
By contrast, pentazocine, an agonist-antagonist opioid (i.e., an agent that is both an agonist at kappa receptors and an antagonist at mu opioid receptors), produces dysphoria and psychotomimesis (psychotic symptoms), an effect that most likely is mediated via kappa2 receptor agonism.10 Pentazocine also can induce a withdrawal syndrome in opioid-tolerant individuals secondary to its mu opioid receptor antagonist effects. For these reasons, many patients previously exposed to pentazocine will cite allergies to it.
Respiratory Depression
All opioid agonists produce dose-dependent depression of ventilation. At equianalgesic doses, all opioid agonists lead to a similar degree of respiratory depression.11,12 In the absence of secondary causes, death from opioid overdose is almost exclusively caused by respiratory depression.
Medullary mu2 receptors are thought to be responsible for the development of respiratory depression. Stimulation of these receptors diminishes chemoreceptor sensitivity to hypercapnia, resulting in loss of hypercarbic ventilatory stimulation.13 Activation of these receptors also decreases the central response to hypoxia13 and inhibits the medullary and pontine respiratory centers that regulate the rhythm of breathing.12 The combination of these effects leads to prolonged pauses between breaths, periodic breathing, hypopnea, bradypnea, and in extreme cases, apnea. It is important to note that the initial manifestation of respiratory depression may be a hypopnea, with or without a decrease in respiratory rate.12
Patients do not develop complete tolerance to the respiratory depressant effects of the opioids.14 For example, patients enrolled in methadone maintenance therapy can experience chronic hypoventilation and hypercapnia.15 A ceiling effect on respiratory depression exists with partial agonist and agonist-antagonist opioids such as nalbuphine and buprenorphine.
Certain groups of patients are particularly sensitive to the ventilatory depressant effects of opioids. These groups include the elderly, patients with chronically elevated PaCO2 (e.g., some patients with chronic obstructive pulmonary disease [COPD]), and patients with a depressed level of consciousness for other reasons. A strong painful stimulus sometimes can transiently overcome or prevent respiratory depression. Similarly, during procedural sedation (e.g., for orthopedic reductions) when pain is relieved, respiratory depression can become apparent. Bronchoconstriction also can occur, most likely as a result of histamine release as well as indirect effects on bronchiolar smooth muscle. Depression of ventilation also can occur in patients receiving neuraxial opioid administration; these effects may be delayed and may be accompanied by respiratory depression (see “Neuraxial Opioids”).
Seizures
Seizures are rare with therapeutic use of most opioids, the primary exception being tramadol. If seizures occur in the setting of an acute opioid overdose, hypoxia is likely the cause. Seizures are associated with meperidine, propoxyphene, and tramadol toxicity. These drugs are further discussed in a later section. In a mouse model, naloxone antagonized the convulsant effects of propoxyphene, but not those of meperidine or its metabolite, normeperidine.16 Fentanyl-induced myoclonus can resemble seizure activity, but true seizures are rarely caused by fentanyl.17
Musculoskeletal Effects: Truncal Rigidity and Movement Disorders
Intravenous (IV) administration of opioids has been associated with motor abnormalities ranging from increased tone to overt myoclonus and involving the chest wall and other truncal muscles. This complication is seen when large doses of highly lipophilic opioids such as fentanyl, sufentanil, remifentanil, or alfentanil are administered rapidly by the IV route.18 Whereas it was previously thought that opioid actions at the level of the spinal cord were responsible for this effect, it now appears that a central dopaminergic effect may be contributory. Both naloxone and neuromuscular blockade can overcome rigidity. Vocal cord spasm, although rare, can cause closure of the vocal cords, leading to difficult bag-valve-mask ventilation. As noted, myoclonic activity resembling seizure activity has been observed in patients after being rapidly infused with large doses of fentanyl.17 Serotonin syndrome, characterized by coarse tremors, increased muscular tone, myoclonus, agitation, and autonomic instability, has been associated with the use of both meperidine and dextromethorphan in combination with other serotonergic agents.
Cardiovascular Effects
The peripheral arterial and venous dilation caused by opioids appears to be mediated by both central depression of vasomotor centers and histamine release.19 Hypotension occurs more frequently in stressed individuals and in those with decreased intravascular volume. Histamine release occurs via non–immunoglobulin (Ig)E-mediated mast cell degranulation.20 Different opioids produce different degrees of histamine release; for example, meperidine and morphine produce much greater release of histamine than fentanyl and sufentanil.21 The severity of histamine-mediated responses can be reduced by slowing the rate of infusion, and hypotension can be reduced by optimizing intravascular volume. Use of Trendelenburg position and saline infusion are appropriate initial interventions for opioid-associated hypotension.
Overall, there are no consistent effects of opioids on cardiac output or the electrocardiogram (ECG). Wide-complex dysrhythmias and impaired contractility are associated with propoxyphene overdose via sodium channel blockade (class Ia antidysrhythmic effect). Illicit opioid use sometimes is associated with cardiac effects secondary to adulterants or co-ingestants; examples are quinine and cocaine (“speedball”). Chronic high-dose methadone use is associated with prolongation of the QT interval.22
 Specific Agents
Specific Agents
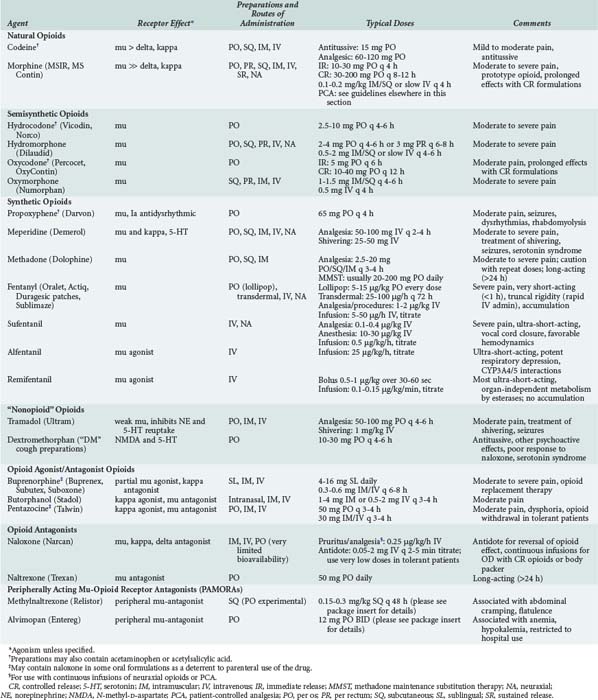
Opioids are among the most widely used drugs in clinical practice. A comprehensive knowledge of their effects and therapeutic applications is essential for any intensive care provider. Table 184-3 summarizes specific agents used in clinical practice.
Heroin
Heroin, also referred to as diacetylmorphine, is a highly lipophilic, semisynthetic opioid produced by acetylation of morphine. Heroin is a prodrug and is devoid of intrinsic opioid effects. It rapidly enters the CNS, where it is deacetylated to the active metabolites, monoacetylmorphine and morphine. Illicit heroin is typically administered by nasal insufflation, SQ injection (i.e., “skin popping”), smoking, or IV injection. The practice of inhaling vapors from heroin heated in aluminum foil is termed “chasing the dragon”; it is associated with a rapidly progressive, irreversible spongiform leukoencephalopathy.23–25
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree