95 Nutrition Issues in Critically Ill Children
Recent reviews of the literature on nutritional support for critically ill children details the lack of definitive or reliable studies to guide our practice based upon scientific evidence.1,2 Therefore, many of the recommendations made rest upon “good practice” principles which rely upon expert consensus and avoidance of known harm whenever possible. The American Society of Parenteral and Enteral Nutrition (A.S.P.E.N.) has promulgated a set of expert guidelines for supporting critically ill children that represent a reasonable standard of care for children in the pediatric intensive care unit (PICU).3
 Impact of Physiologic Stress on Children
Impact of Physiologic Stress on Children
Alterations in protein and energy metabolism are hallmarks of critical illness and have been studied for many decades.4 This work has demonstrated a great difference between short-term starvation states in otherwise healthy individuals and the dramatic “autocannibalism” seen in critically ill patients who are not receiving appropriate nutritional support as summarized in Table 95-1.
TABLE 95-1 Comparison of Nutrient Metabolism in Starvation Versus Sepsis/Trauma
| Starvation | Sepsis/Trauma | |
|---|---|---|
| Protein breakdown | ++ | +++ |
| Hepatic protein synthesis | ++ | ++++ |
| Ureagenesis | ++ | ++++ |
| Gluconeogenesis | ++ | ++++ |
| Energy expenditure | Reduced | Increased |
| Mediator activity | Low | High |
| Hormone counterregulatory capacity | Preserved | Poor |
| Use of ketones | +++ | + |
| Loss of body stores | Gradual | Rapid |
| Primary fuels | Fat | Amino acids, glucose, triglycerides |
Adapted from Barton R, Cerra FB. The hypermetabolism-multiple organ failure syndrome. Chest 1989;96:1153-60.
The events that lead to ICU admission are extremely varied, yet the body’s response to acute physiologic stress tends to be similar whether the inciting event is sepsis, ischemia-reperfusion, trauma, burns, or other inflammatory conditions. Beyond low levels of stress, such as minor elective surgery, life-threatening illness, burns, organ transplantation, or major surgical procedures elicit dramatic systemic inflammatory responses due to activation of the immune system, clotting mechanisms, and the endothelium. The patient’s ability to withstand the metabolic responses to such stresses and ultimately to reverse the process is central to recovery. A complete discussion of the metabolic response to stress is beyond the scope of this chapter; the reader is referred to other sources.5,6
The initial response to injury is to activate endothelial cells and to prime inflammatory cells such as neutrophils, macrophages, and lymphocytes through proinflammatory mediators including tumor necrosis factor, interleukin 2, histamine, eicosanoids, heat-shock proteins, free radicals, platelet-activating factor, and tryptases.7 These same signals that produce activation of the endothelium lead to permeability changes, activation of clotting mechanisms, and changes in hepatic and peripheral protein metabolism.8 If recovery is to occur, this process must be extinguished by a decrease in the inflammatory state and an increase in tissue repair.9 Although it may seem that simply shutting off the proinflammatory signals should lead to resolution, the process of resolving inflammation appears more complex.10 Studies show the importance of many of the proinflammatory stimuli in regeneration and repair, and the timing of interventions is important.11 In response to injury, a wide range of neurohumoral reactions occur, forming the classic “stress response,” which includes elevation of growth hormone, endogenous catecholamines, glucagon, and cortisol. Recognition of the role of insulin-like growth factor-1 along with growth hormone in promoting protein synthesis and counter-regulating inflammatory states suggests important potential treatment options that have been best studied in burns. Despite these studies showing benefit from growth hormone supplementation, evidence of increased mortality rate after growth hormone supplementation also has been reported.12 Clinicians must balance the relative benefit of hormonal manipulation with potential risks.
In the inflammatory state, unremitting gluconeogenesis occurs through the release of glycerol and gluconeogenic amino acids from the periphery with their conversion to glucose in the liver and kidney. Hyperglycemia frequently is associated with this state and may induce glycosuria and an osmotic diuresis. Insulin activity becomes impaired at the tissue level, leading to so-called insulin resistance in the face of the powerful gluconeogenesis driven by the stress hormones. It seems that the impairment of insulin results from decreased phosphorylation of the insulin receptor and second messengers.11 In the last decade, evidence from adult ICU experience has suggested a benefit from the use of insulin infusions to maintain tight control over serum glucose level.13 Although much of the preceding information derives from adult studies, it has found its way into contemporary pediatric practice in many centers in children of various ages. This question is receiving intense scrutiny in critically ill children through multicenter trials which are currently underway. The use of insulin infusions to control hyperglycemia in premature infants continues to be standard practice; however, the potential to produce marked hepatic steatosis under the influence of insulin should be born in mind when choosing the amount of carbohydrate to provide.
The breakdown of protein is a central theme in the body’s response to stress, which has wide-ranging significance beyond simple protein losses. The conversion of certain amino acids to glucose and the oxidation of others in peripheral tissues lead to the liberation of large quantities of amino-nitrogen, which would become toxic if not for the efficient conversion to urea. A dramatic increase in the rate of urea production is seen in critically ill patients. Concomitantly, other non-urea nitrogen is liberated in the form of uric acid and creatine and accounts for the dramatic increase in nitrogen wasting seen during stress states. Total urinary nitrogen losses in critically ill children may be 0.3 g/kg/d, which represents the loss of approximately 1.8 g/kg/d of whole protein catabolized. In parallel with the increased turnover of proteins, the metabolic rate for oxidation of energy substrates may increase following acute critical illness during the recovery phase (see subsequent section on energy expenditure).
The body’s response to withholding feeding (i.e., starvation) in healthy individuals is qualitatively and quantitatively different than that seen when nutrient intake is absent during critical illness. These differences are fundamental to understanding nutritional support in the ICU and are summarized in Table 95-1. In simple starvation, the body’s regulatory mechanisms for sparing lean tissue and using triglycerides as the primary energy source are intact, whereas under the influence of the stress response, rapid depletion of lean tissues occurs with oxidation of amino acids, carbohydrate, and fat as energy substrates.
One of the major consequences of life-threatening physiologic stress is the net depletion of body protein representing the somatic protein pool (e.g., skeletal muscle mass) and functional (e.g., plasma proteins, enzyme systems, antibodies) tissues contained in the visceral protein pool. With protein catabolism rates increased up to twofold, synthesis does not keep pace, and a state of negative nitrogen balance ensues when patients are not given adequate calories and protein.14 These changes produce depressed function of T and B lymphocytes, monocytes, and neutrophils as cumulative protein loss increases. The synthesis of antibodies, chemotaxis, phagocytosis, and bacterial killing is impaired in the face of advanced protein-calorie malnutrition.15 A decrease in total lymphocyte count may be seen in many patients, but a total lymphocyte count less than 1200/mm3 should raise concern for the presence of possible immune dysfunction. These alterations lead to impairment of host defense mechanisms. As noted earlier, for resolution of the inflammatory response, the patient’s immune system plays a central role in recovery of wound healing and recovery of immune competence.16 It is likely that the syndrome of multiple organ dysfunction seen in critically ill patients is due in part to the inability of the immune system to down-regulate the inflammatory response to injury in specific organs, as well as acquired mitochondrial dysfunction leading to ineffective cellular energy production.17 Nutritional support of a critically ill patient is thought to be essential to achieving recovery and minimizing the subsequent period of convalescence.
Considerable attention currently is focused on the use of modified nutritional support regimens in critically ill adults to modify the inflammatory response and reduce secondary organ system dysfunction.18 A wide range of substances have been studied in an attempt to improve outcome or minimize nitrogen loss during critical illness in specific populations of patients. Glutamine supplementation appears to benefit critically ill adults, particularly those with burns.18 Omega-3 fatty acids appear to also be beneficial in patients with sepsis and systemic inflammatory response syndrome (SIRS). The results in adults suggest that formulas supplemented with these products improve oxygenation and reduce the alveolar inflammatory response during acute respiratory distress syndrome (ARDS). While trials of these agents are underway in critically ill children, there is still not a strong enough consensus among pediatric specialists to consider their use as standard therapy.3
 Nutrition Assessment
Nutrition Assessment
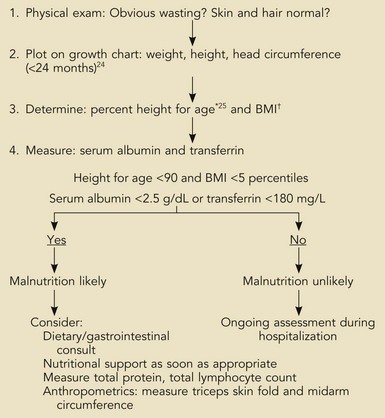
The nutrition assessment of hospitalized children is a central and critical part of the initial examination and evaluation of all patients. The existence of chronic malnutrition as well as the development of acute malnutrition during critical illness has been recognized in pediatric critical care for many years19–21 and appears to be an unmet need even today.22 Therefore, clinicians must assess newly admitted patients for the presence of malnutrition that may complicate the response to therapies or impair recovery (Box 95-1). The presence of previous severe malnutrition may complicate critical care management through the presence of marasmic cardiomyopathy, severe intracellular energy deficiency, and the development of refeeding disequilibrium when nutrients are provided in the ICU.23
Box 95-1
Assessment of Nutrition Status on Admission
*Actual height (cm) × 100/expected height at 50th percentile for age.
†BMI (kg/m2) = body mass index: actual weight (kg)/[actual weight × height (m)] 2
From Statistics NCfH. CDC growth charts, United States, 2000. Available at: http://www.cdc.gov/growthcharts; and from Waterlow J. Classification and definition of protein-calorie malunutrition. Br J Med 1972;3:566-9.
The initial nutrition evaluation consists of assessing the patient’s weight, height, historical evidence for recent weight loss, and anthropometric measurements including midarm circumference and skinfold determination (when edema is not present). Nutrition history must include the presence and duration of nausea, vomiting, diarrhea, fever, frequent infections, fatigue, food aversion, abdominal discomfort, or feeding intolerance. For growth standards, norms exist reflecting age and gender.24 Ethnic background and considerations such as the presence of certain syndromes (e.g., Down syndrome) or the child’s birth status (e.g., premature, growth restricted, etc.) may affect the child’s growth status.
In particular, determination of body mass index (BMI, previously known as weight-for-height) for children older than 2 years of age provides important information regarding the previous nutritional status (see Box 95-1). In children younger than 2 years, the weight-for-age in light of the previous growth status is most useful. These straightforward measurements have withstood the test of time and were used by Pollack and coworkers to estimate the risk of malnutrition in critically ill children admitted to a multidisciplinary PICU.19,20,25 Their findings demonstrated higher rates of preexisting malnutrition than had been previously thought. In addition, there was an unexpected deterioration in nutrition indices following admission, suggesting the powerful effects of life-threatening illness on nutritional stores and status even with excellent clinical care. Clinicians caring for children who will experience more than a few days of hospitalization must therefore be especially aware of the potential for acquired nutritional depletion. Potential sources of error exist in interpreting anthropometric measurements that are primarily related to changes in body water associated with many acute critical illnesses in children (i.e., conditions producing capillary leak syndrome or defects in renal water clearance). Such conditions may invalidate the measurement of skinfold or midarm circumference; however, their longitudinal use in patients can be very useful in estimating the accretion of fat and lean tissue stores. It is standard practice to measure these parameters in patients at risk for malnutrition, such as those with cystic fibrosis, short bowel syndrome, and other conditions in which malabsorption or chronically elevated metabolic demands exist (e.g., congenital heart failure, bronchopulmonary dysplasia, and similar chronic conditions).
The triceps and scapular skin folds measure the subcutaneous tissue compartment (consisting primarily of adipose tissue) but also tissue edema in patients with anasarca from any cause. Triceps skin fold is measured by standardized skin caliper and is subject to considerable error if not performed in a consistent manner midway between the acromion and olecranon. The midarm circumference should be measured at the same point with a nonstretchable tape measure. The two indices taken together permit a reliable estimate of muscle mass. In general, good correlation exists between skinfold and arm circumference and weight-for-height percentile.26 During critical illness, anasarca may obscure the loss of lean tissue, which may only be apparent following resolution of edema when successful diuresis has occurred. A very reliable indicator of global loss of lean body mass can be seen in the wasting of the interosseous and thenar muscles of the hand, which becomes apparent 2 or 3 weeks after hospitalization with resolution of edema.
Shorter half-life serum proteins such as prealbumin [T1/2 = 2 days] and transferrin [T1/2 = 7 days] also reflect nutrition status and respond more quickly to changes in anabolic state.27 As noted earlier, the pool of proteins in the plasma, interstitial space, and some intracellular proteins represent a relatively labile pool of protein referred to as the visceral protein pool. Visceral proteins are rapidly turned over relative to structural proteins that comprise the somatic protein pool. In critical illness, the synthesis of specific proteins such as C-reactive protein, ceruloplasmin, and α2-macroglobulin is increased, whereas the synthesis of other proteins such as albumin [T1/2 = ~20 days] is decreased.28 These changes may be seen within 6 hours of the onset of severe physiologic stress. This response to physiologic stress is under the regulation of complex neurohumoral control and is referred to as the acute phase response. It is largely responsible for the increase in erythrocyte sedimentation rate associated with acute inflammatory conditions.29 When followed longitudinally, the return of previously depressed levels of certain visceral proteins such as albumin, transferrin, retinol-binding protein, or prealbumin represents the abatement of physiologic stress or improvement in nutrition when levels are low due to protein-calorie malnutrition. Such positive changes herald the impending return to a state of growth and tissue accretion, barring reentry into a new inflammatory state.