Ireton-Jones Energy Equations (IJEE) 1992
SB: REE = 629 – 11(A) + 25(BW) – 609(O)
VD: REE = 1925 – 10(A) + 5(BW) + 281(S) + 292(T) + 851(B)
S, sex (male = 1, female = 0), T, trauma (present = 1, absent = 0), B, burn (present = 1, absent = 0),
(O) BMI > 27 kg/m2 (present = 1, absent = 0).
Mifflin-St. Jeor
M: REE = (9.99 × BW) + (6.25 × H) – (4.92 × A) + 5
F: REE = (9.99 × BW) + (6.25 × H) – (4.92 × A) – 161
Penn State Equation
(PSU 2003b)
REE = Mifflin (0.96) + VE (31) + Tmax (167) – 6212
Used for patient of any age with BMI <30 or patients < 60 years with BMI > 30.
Penn State Equation
(PSU 2010)
REE = Mifflin (0.71) + VE (64) + Tmax (85) – 3085
Used for patients with BMI > 30 and older than 60 years.
Fasy-Fagon
REE (kcal/d) = 8 × BW + 14 × H + 32 × VE + 94 × T – 4,834
American College of Chest Physicians (ACCP)
REE = 25 × BW
If BMI 16–25 kg/m2, use usual BW
If BMI > 25 kg/m2, use IBW
If BMI < 16 kg/m2, use ABW for the first 7–10 days, then use IBW
ESPEN Guidelines
20–25 kcal/kg BW/d during the acute and initial phase of critical illness
25–30 kcal/kg BW/d during the anabolic recovery phase
Energy requirements of hospitalized COPD patients were estimated at 30 kcal/kg/day. A reduction in mechanically assisted patients is likely due to decreased work of breathing [10]. For stable, high-level quadriplegic patients, the Evidence-Based Nutrition Guidelines for Spinal Cord Injury recommend energy intake equal to 22.7 kcal/kg/day. Diminished metabolic requirements in these patients are attributed to lower limb paralysis. It is difficult to define energy requirements in patients with amyotrophic lateral sclerosis (ALS) because, although ALS leads to skeletal muscles’ atrophy, patients are hypermetabolic. A general approach is the following:
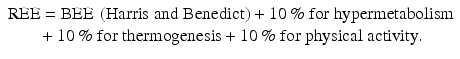 Vaisman et al. [11] propose an equation that predicts the actual measurement of REE in 86 % of its variability. It can be used only in malnourished patients with documented reduced caloric intake compared with the predicted value and should be recalculated along the disease course.
Vaisman et al. [11] propose an equation that predicts the actual measurement of REE in 86 % of its variability. It can be used only in malnourished patients with documented reduced caloric intake compared with the predicted value and should be recalculated along the disease course.
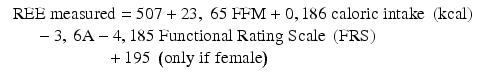 Obese patients deserve special attention. According to ESPEN and American Society for Parenteral and Enteral Nutrition (ASPEN) guidelines, energy intake should be restricted to 11–14 kcal/kg of actual BW (ABW) or 22–25 kcal/kg of ideal BW (IBW).
Obese patients deserve special attention. According to ESPEN and American Society for Parenteral and Enteral Nutrition (ASPEN) guidelines, energy intake should be restricted to 11–14 kcal/kg of actual BW (ABW) or 22–25 kcal/kg of ideal BW (IBW).
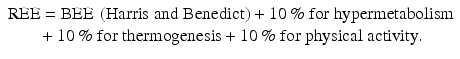
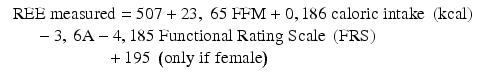
Taking into consideration that all of the above equations may be inaccurate on an individual basis, physicians need to continuously reassess patients’ metabolic demands under close monitoring and readjust their prescriptions when signs of harmful under- or overfeeding are recognized.
There is a growing interest in the importance of adequate protein supply on patients’ outcomes. Adequate protein delivery was proved beneficial for patients by reducing mortality, time on ventilator, and length of stay, and by improving quality of life [12, 13]. There is some evidence that increased protein intake improves physical function post ICU and the rate of discharge to home versus to a rehabilitation center [14]. Protein should be administered in amounts ranging from 1.2 to 1.5 g/kg/day and could be increased to 2 g/kg/day in case there is additional nitrogen loss, as in renal replacement therapy, decubitus ulcers, or high-output drainage. For obese patients, more than 2 g/kg of IBW is suggested. There are no studies proving that enrichment of diets with specific amino acids has any beneficial effect in muscle protein synthesis or the weaning process.
The interrelation between substrate oxidation and ventilation was the basis of the controversy regarding the appropriate composition of the nonprotein calories of a diet. Earlier studies supported the use of low-carbohydrate (CHO), high-lipid diets to reduce CO2 production in MV patients, whereas later ones proved that hypercapnia was the consequence of the combination of overfeeding and CHO load rather than the CHO load alone. In addition, high-lipid diets were found to cause delayed gastric emptying and intolerance to enteral nutrition (EN). Because of these conflicting results and the impact of the high-lipid diets on gastric emptying, there are no recommendations on the ordinary use of low-CHO, high-lipid diets in LTMV patients [9, 15].
LTMV patients can be at risk for micronutrient deficiencies; however, because there is no reported data on this prevalence, routine provision should be restricted to recommend daily allowances, except when clinical signs of specific deficiencies are diagnosed. Low levels of 25(OH) D have been reported in studies with LTMV patients, raising concern about vitamin D. Investigators attributed the high rate of bone hyper-resorption in 92 % of CCIP to low levels of 25(OH) D, along with immobilization. Authors suggest that low levels of 25(OH) D should be treated with a combination of calcitriol and pamidronate, together with the recommend daily allowances for Ca [9]. Low serum P levels should be repleted, inasmuch as hypophosphatemia can lead to respiratory insufficiency and weaning failure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




