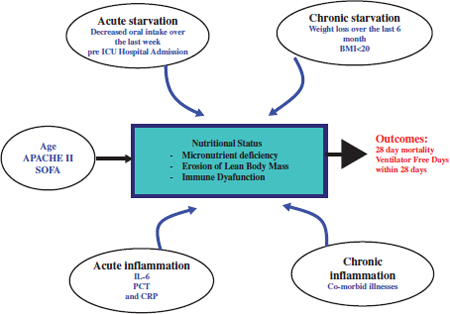
Chapter 6 Many patients will require some form of nutrition during their stay in the intensive care unit (ICU) due to inability to eat, increased metabolic needs, and physiologic stress. In this chapter, we discuss the physiologic response to stress and starvation, assessment of the nutritional status of the patients and the energy requirements, which will determine their nutritional needs. Enteral Nutrition (EN) is the mode of choice where possible, parenteral nutrition being reserved for other patients in whom EN cannot be provided. In the Surgical ICU, patients are usually kept nil per OS for a variable duration. For brief period of 2–3 days, nutritional supplements are not a major issue but patients in the ICU for prolonged periods require nutrition support. Restoration of glucose levels for organs dependent on glucose as a fuel (brain, erythrocytes, and kidney) occurs initially by hepatic glycogenolysis which is short lived. As levels of insulin fall and glucagon rises, lipolysis in adipose tissue and proteolysis in skeletal muscle stimulate gluconeogenesis in the liver. At the same time, peripheral tissues switch to utilization of fatty acids and ketones to produce Acute Thrombocytopenic Purpura (ATP). After about one week of continuous fasting, the brain adapts to use ketones for 50% of its fuel needs. Gluconeogenesis and muscle breakdown then slow down dramatically.1 During physiologic stress (e.g., Trauma, Burn, sepsis), catecholamines, glucagon, cortisol, and cytokines (IL-1, IL-6, TNF-α, and Interferon-γ,) are released, causing major physiologic changes. In skeletal muscle, protein breakdown continues with decreased protein synthesis. As a result, amino acid pools become depleted. Similar changes occur in intestinal mucosa with glutamine becoming an essential amino acidare. Amino acids are delivered to areas where protein synthesis continues (Lungs, heart, spleen, and liver). In the Liver, alanine is utilized in gluconeogenesis and synthesis of acute phase reactant proteins. This gluconeogenesis is not reversible by hyperglycemia or administration of glucose. A state of insulin resistance results in hyperglycemia. Lipolysis also occurs in this highly catabolic phase. These factors should be considered when assessing the nutritional needs.1,2 Determination of the nutritional status as well as the energy expenditure are important in planning nutritional support. The most commonly used formula for nutritional requirements is weight based and estimates the caloric requirement at 25–35 Kcal/Kg/day.2 Harris–Benedict Equations are also used as follows: Male: Basal Energy Expenditure (BEE) = 66.5 + (13.8 × weight in kg) + (5 height in cm) − (6.8 × age) Female: Basal Energy Expenditure (BEE) = 66.5 + (9.6 × weight in kg) + (1.7 × height in cm) − (4.7 × age) BEE is then multiplied by activity factors or stress factors.2 Indirect Calorimetry measures oxygen consumption and Carbon dioxide production and provides values on measured energy expenditure using the Respiratory Quotient (RQ), which is the ratio of O2 consumption to CO2 production. RQ between 0.9–1 indicates Carbohydrates, 0.8–0.9 indicates proteins, 0.7–0.8 indicates fat as the main fuel. Values >1.1 suggest overfeeding, and values <0.7 suggests ketogenesis.1,2 One pilot randomized trial comparing feeding targeted by indirect calorimetry to the weight-based formula showed a trend toward improved survival with indirect calorimetry.3 However, achieving a target dose of enteral nutrition, by starting at a target dose, accepting higher threshold of gastric residual volumes, use of prokinetic agents and small bowel feeding, collectively as a bundle may change patient outcome.4 Intentional trophic feeding which involves fixed small volumes feeding such as 10ml/hour for the first six days has been studied in two randomized trials in patients with acute lung injury. Trophic feeding did not improve ventilator-free days or 60-day mortality but had fewer episodes of gastrointestinal intolerance.5,6 Hypocaloric intake has been compared to full caloric intake in one randomized trial, and there was a trend toward improvement in survival. A larger trial (Permit Trial) is ongoing and may answer this clinical question.7 Recently, a conceptual model has been described (see Fig. 1) involving several factors affecting the nutritional status of the critically ill. Using these factors, a novel score has been described and validated. The score is described in Table 1. Higher scores have been correlated with worse clinical outcomes (mortality and ventilation days) and these patients with such scores will most likely benefit from aggressive nutritional therapy.4,8 Fig. 1. A conceptual model for nutrition risk assessment in the critically ill. APACHE, Acute physiology and chronic health evaluation score, BMI, body mass index, CRP, C-reactive protein, IL-6, interleukin 6, PCT, procalcitonin, SOFA, sequential organ failure assessment score. (With permission from Dr. D. Heyland, Heyland et al. Crit Care 2011; 15(6)).8 0–<400 0 In the presence of IL-6: A score between 6 and 10 is associated with worse clinical outcomes and these patients most likely will benefit from aggressive nutrition. A score of 0–5, these patients have a low malnutrition risk. In the absence of IL-6: A score between 5 and 9 is associated with worse clinical outcomes and these patients most likely will benefit from aggressive nutrition therapy. A score 0–4, these patients have a low malnutrition risk. (With permission from Dr. D. Heyland, Heyland et al. Crit Care. 2011; 15(6)). Generally, every effort should be made to use the gut and institute enteral nutrition (EN) rather than parenteral (PN). There is strong evidence that EN is associated with significant reduction of infectious complications and is more cost effective.4 However, there was no difference in mortality or in hospital length of stay. EN feeding should be started early within 24–48 hours following ICU admission. Early EN is associated with lower mortality and lower infectious complications. Although there is no difference in length of stay nutritional goals are better achieved with early EN.4 Many patients in the surgical ICU are hemodynamically compromised and some may require vasopressors. Institution of early EN in these patients is controversial. During hypotension blood will be shunted from the gut to more vital organs (heart and brain). Vasopressors increase the blood pressure but increase risk of gut ischemia due to vasconstriction. After volume resuscitation, blood flow to the gut will not be restored immediately because of concomitant increase in endogenous vasoconstrictors (endothelin I, angiotensin II, and vasopressin) and decrease in endogenous vasodilators (nitric oxide). However, EN will increase blood flow to the gut. Two studies in cardiac surgery patients showed that EN is feasible, safe and not associated with major complications. In severely burned patients, early feeding within 48 hours did not increase CO2 gap and it was well tolerated. A retrospective study in septic patients showed early EN had some delayed gastric emptying. In a large observational study in patients on vasopressors and mechanical ventilation, early EN was associated with lower hospital mortality. This study suffers from selection bias where patients with poor ICU status received delayed nutrition. These studies suggest that early EN is feasible and may not be associated with serious complications but a large randomized trial is warranted.9 In the short term, nasoenterirc tubes (gastric, duodenal, or jejunal) may be used for EN. The large tubes (16 or 18-F) that come with the patient from the operating room may be used for feeding as well. They allow measurement of gastric residuals and do not clog easily. They can be uncomfortable and theoretically increase reflux. The small caliber tubes (e.g., stylet-type) allow advancement into the duodenum or jejunum (under fluoroscopic- or endoscopic-guidance) and can be used for feeding in patients with poor gastric emptying (high gastric residuals or with gastroparesis). Usually tubes placed in the small bowel require continuous feeding and those placed in the stomach allow bolus feeding. Complications include malposition in the bronchus with potential feeding into the lung. A chest X-Ray (CXR) showing the tube below the diaphragm is therefore mandatory before institution of EN. Sinusitis due to nasally placed tube can be a cause of fever in the critically ill. Other complications include tube migration, esophageal and gastric mucosal erosions, pulmonary aspiration, pneumothorax, esophageal stricture, esophageal perforation, and fatal dysrrhythmias.1,10 For the long-term, gastrostomy or jejunostomy tubes should be used. Gastrostomy tubes can be inserted via a small laparotomy incision (the Stamm Gastrostomy), endoscopically (so-called Percutaneuos Endoscopic Gastrostomy (PEG) tube) or percutaneuosly by interventional radiology. PEG tubes are less expensive with lower complications than conventional Stamm Gastrostomy. In patients with previous upper abdominal surgery, the blind percutaneuos technique is contraindicated because of possible adhesions and inadvertent insertion into the colon. In experienced hands, laparoscopic-assisted PEG tube insertion is a good option, during which the laparoscope guides insertion of the needle into the stomach.11 Another option is open Stamm Gastrostomy via a left upper quadrant incision.1,10 Jejunostomy tube may be inserted surgically via open or laparoscopic techniques. It may also be inserted endoscopically by extension of the existing gastrostomy (called G-J tube) or percutaneuosly by interventional radiology. The G-J tube has a double lumen with an advantage of decompression of the stomach during small bowel feeding. The J tube is often required in patients who are not tolerant of gastric feeding (e.g., Gastroparesis). In the critically ill patient, hypo-osmolar or iso-osmolar feeding should be used in a continuous fashion rather than bolus feeding. Small bowel does not tolerate bolus feeding and hyper-osmolar feeding is associated with pneumatosis and necrosis.1,10 Multiple trials compared gastric and small bowel feeding and consistently showed that small bowel feeding is associated with a reduction in pneumonias. Therefore, small bowel feeding should be routinely used especially in patient not tolerating gastric feeding (e.g., on vasopressors, high doses of sedatives or paralytic agents, and high gastric output) or patients at high risk of regurgitation and aspiration (e.g., nursed in supine position).4 One small trial compared early PEG tube insertion to nasogastric feeding in patients with stroke and head injury. This trial included only 41 patients and showed lower incidence of ventilator-associated pneumonia with early PEG tube insertion.12 Because of lack of strong evidence at this time, this approach is not recommended. EN can be administered in the critically ill either continuously or intermittently. Continuous administration is usually started at a low rate of 20 cc/hr and increased by 20 cc increments every 8–12 hours until the goal rate is achieved. Intermittent administration (or bolus feeding) is given by 100–125 cc bolus by gravity over 15 minutes every 4–8 hours and increased by increments of 100–125 cc every 8–12 hours. Multiple trials compared the two techniques and showed no difference in mortality, infections, length of stay, frequency of interrupted feeds, percent of goal feeds achieved, or diarrhea.4 • Protein versus peptides Peptide-based formula has been compared with whole-protein formula in several randomized trials. The hypothesis is that introduction of simple peptides are better absorbed in the critically ill and produce less diarrhea. These studies demonstrated no difference in mortality, infection rate and length of stay with varying effects on diarrhea. Despite the higher cost of peptidebased formula, however, certain groups of patients (e.g., short-bowel syndrome and pancreatitis) may benefit from them.4 • Feeding protocols The use of prokinetics is usually reserved for patients who are intolerant of gastric feeding with high gastric residuals. Concomitant prokinetics use with small bowel feeding have shown a trend toward a reduction in hospital mortality and reduction of hospital length of stay but not the ICU length of stay.13,14
Nutrition in the Surgical ICU Patient
Mohammed Bawazeer and Jameel Ali
Chapter Overview
Physiologic Adaptation to Fasting States, Stress, and Sepsis
Nutrition Assessment
Variable
Range
Points
Age
<50
50–<75
≥75
0
1
2
APACHE II
<15
15–<20
20-28
≥28
0
1
2
3
SOFA
<6
6–<10
≥10
0
1
2
Number of co-morbidities
0–1
≥2
0
1
Days from hospital to ICU admission
0–<1
≥1
0
1
IL-6
≥400
1
General Principles
Enteral Nutrition
A. Routes of Administrations
B. Practical Issues
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree