Nitric Oxide as an Adjunct: Introduction
As the saying goes, unexpected scientific discoveries are often the most important. The “principle of limited sloppiness,” a term coined to describe fortuitous or accidental discoveries, hit in the 1970s, when Zawadski, a technician in the laboratories of Robert F. Furchgott, failed to follow his superior’s directions correctly and did not remove the endothelium in a rabbit aorta preparation. In this preparation, acetylcholine caused potent relaxation whereas contraction was expected. Shortly thereafter, it was established that acetylcholine was acting on endothelial cell receptors to produce a substance that could diffuse to the vascular smooth muscle and initiate its relaxation.1 This substance was called endothelium-derived relaxing factor. It took another 8 years for independent working groups to confirm that the chemical structure of endothelium-derived relaxing factor was identical to that of nitric oxide (NO).2,3
The scientific and global community honored the substance itself and its discovery by naming NO “Molecule of the Year” in 1992.4 The Nobel Prize in Physiology or Medicine for 1998 was awarded jointly to Robert F. Furchgott, Louis J. Ignarro, and Ferid Murad, for their breakthroughs concerning “nitric oxide as a signaling molecule in the cardiovascular system.”
NO is a colorless and odorless gas. It is a toxic air pollutant, present in motor vehicle exhaust and power plant effluent. The gas is found in the atmosphere in the range of 10 to 500 parts per billion (ppb), and locations with heavy vehicular traffic can exceed 1.5 parts per million (ppm). In the hot cone of a glowing cigarette, concentrations of 1000 ppm were measured in a 40-mL puff. NO is a free radical; it quickly reacts with oxygen (O2) to form poisonous nitrogen dioxide.
In the 1980s it became evident that NO is an essential molecule that regulates a wide range of human physiologic processes. Early studies revealed that NO is produced in endothelial cells and diffuses to vascular smooth muscle cells, where it mediates relaxation. Further studies demonstrated that the substance controls several other physiologic systems, including the immune system, platelet aggregation, and neurotransmission. The focus of this chapter is the prominent role of NO in respiratory physiology and its therapeutic application by inhalation.
Endogenous Nitric Oxide in the Respiratory System
Endogenous NO is produced by the enzyme system, nitric oxide synthase (NOS). In human subjects, NOS activity can be found in the epithelium of nasal and paranasal mucosa, the bronchial epithelium, type II alveolar epithelial cells, airway nerves, inflammatory cells, airway and vascular smooth muscle cells, and endothelial cells. Three isoforms of the enzyme have been identified: the constitutive neuronal NOS, the inducible NOS (iNOS) that is incited by cytokines, and the constitutive endothelial NOS. There is evidence that a fourth isoform, mitochondrial NOS exists, which has important functions in cellular metabolism.
NO generated by neuronal NOS in the peripheral nervous system acts as a neurotransmitter that modulates smooth muscle relaxation in the respiratory tract. Inflammatory cells express iNOS that enhances NO synthesis. It is supposed that NO plays a self-regulatory role in host-defense and inflammatory processes. Yet, there are conflicting results whether NO mediates proinflammatory or antiinflammatory effects. Concurrently, overproduction of NO may also be associated with the worsening of certain infectious diseases. NO formed by endothelial NOS in vascular endothelial cells regulates pulmonary and systemic vascular tone. Mitochondrial NOS is assumed to provide substantial amounts of cardiac NO responding to heart hypoxia. Figure 61-1 shows the schematic pathway of NO signal transduction.
Figure 61-1
Endogenous or inhaled nitric oxide (NO) mediates vasodilation of vascular smooth muscle cells. NO is endogenously produced in endothelial cells of the pulmonary vasculature from the amino acid l–arginine, which is converted to l-citrulline and NO by the enzyme nitric oxide synthase. NO expressed by the endothelial cells, or inhaled NO, diffuses rapidly into the vascular smooth muscle cells, where it activates soluble guanylate cyclase, which converts guanosine triphosphate (GTP) into cyclic guanosine monophosphate (cGMP). The high intracellular concentration of cGMP relaxes the smooth muscle via cGMP-dependent protein kinase. cGMP is inactivated by the enzyme phosphodiesterase, which catalyzes the conversion of cGMP to guanosine monophosphate (GMP). eNOS, endothelial nitric oxide synthase.
The concentrations of NO in healthy human airways differ depending on the measurement site. Approximately 100 ppb were measured in the nasopharynx of healthy nonsmoking volunteers during nose breathing. During mouth breathing, even higher concentrations of 650 ppb were seen in the nasopharynx. The highest NO concentrations (1 to 30 ppm) were detected in the paranasal sinuses.5 In the trachea of intubated patients the NO concentrations were markedly lower; they ranged between 5 and 10 ppb. The cilia of the epithelial cells of the maxillary sinuses contain high amounts of iNOS and can be viewed as a major production site of NO in the respiratory tract.6
Endogenous NO was suggested to be an important signaling molecule in numerous physiologic processes. Autoinhaled NO from the paranasal sinuses is able to induce selective pulmonary vasodilation in ventilated areas. The blood flow through well-aerated lung areas with higher intraalveolar O2 concentrations increases and ventilation–perfusion mismatch is antagonized.
Endogenous NO is also involved in host defense and has direct microbicidal effects. Especially the high levels of endogenous NO in the paranasal sinuses may be active in local nasopharyngeal host defense against bacterial or viral invaders. Other findings substantiate that endogenous NO increases airway mucus secretion. Ciliary beat frequency, responsible for microbial clearance, is also enhanced by iNOS stimulators.
Bronchodilation is suppressed by NOS inhibitors, indicating that endogenous NO modulates basal bronchial tone. There is evidence that endogenous NO may protect the airways of asthmatic patients from bronchoconstriction.7 The high levels of NO, measured in the exhaled air of patients with asthma, however, are believed to mirror the stimulation of iNOS by proinflammatory cytokines and seem to have harmful effects, such as inflammation and increased vascular permeability.
NO is excreted by tumor and host cells and is a factor of promoting angiogenesis and tumor growth. The role of NO in cancer, however, is yet undetermined as it has been attributed both, tumoricidal as well as tumorigenic properties. There are indications that inhibiting iNOS may be beneficial in treatment of certain forms of cancer.
Furthermore, endogenous NO possibly regulates the coagulation system. Formation of endogenous NO also seems to be involved in mediating pulmonary vasodilation during transition of the pulmonary circulation at birth.
Rationale for the Use of Inhaled Nitric Oxide as an Adjunct
Inhaled nitric oxide (iNO) acts as a selective pulmonary vasodilator. As a gas, it reaches only ventilated alveoli and produces relaxation of the accompanying pulmonary blood vessels. iNO acts by producing vasodilation in well-ventilated lung units and redistributing pulmonary blood flow from unventilated to ventilated regions of the lung. This results in improvement of ventilation–perfusion mismatch and oxygenation, in a decrease of pulmonary vascular resistance and pulmonary artery pressure (PAP), as well as in a lower right-ventricular afterload. The activity of iNO is mainly limited to the area of deposition when lower concentrations of iNO are applied because large amounts of the molecule are inactivated by binding to hemoglobin at the moment NO diffuses into the blood vessel. This explains why iNO has almost no systemic side effects and acts predominantly in the pulmonary circulation (Fig. 61-2).
Figure 61-2
Inhaled NO selectively produces vasodilation in ventilated alveoli. iNO produces vasodilation in well-ventilated lung units and redistributes pulmonary blood flow from unventilated to ventilated lung regions. In patients with ventilation–perfusion mismatch (e.g., patients with acute respiratory distress syndrome), this leads to improvement in oxygenation and reduction of intrapulmonary right-to-left shunt. Selective vasodilation in the pulmonary circulation causes significant reduction of elevated pulmonary artery pressure; thus, iNO is a valuable therapy in patients with all kinds of pulmonary hypertension. Systemic vasodilation does not occur to a noteworthy extent because large amounts of NO rapidly bind to hemoglobin and are thereby inactivated. Hb, hemoglobin; iNO, inhaled nitric oxide; NO, nitric oxide; 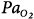 , arterial partial pressure of oxygen; PAP, pulmonary arterial pressure;
, arterial partial pressure of oxygen; PAP, pulmonary arterial pressure; 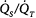 , intrapulmonary right-to-left shunt.
, intrapulmonary right-to-left shunt.
Indications and Outcome of Inhaled Nitric Oxide Therapy
Inhaling low concentrations of NO causes rapid and safe reduction of an elevated PAP, and improves the impaired oxygenation in many patients without causing systemic hypotension. In the early 1990s, iNO became an innovative treatment option for patients with acute respiratory failure and pulmonary hypertension.
In patients with acute respiratory distress syndrome (ARDS), improvement of oxygenation and reduction of PAP is an important therapeutic goal. iNO selectively enhances perfusion in ventilated lung areas and counteracts the ventilation–perfusion mismatch typical of this condition. Because NO works only in aerated lung tissue, measures that recruit previously collapsed alveoli can enhance the beneficial effect of NO. The overall effect of iNO is often an impressively increased arterial partial pressure of oxygen (PAO2) and a reduction of the elevated PAP. Several enthusiastic reports encouraged the hope that iNO would prove to be the promising new therapy that would ultimately improve the low survival rates in ARDS. Disappointingly, an improved outcome of ARDS patients could not be observed. A meta-analysis of twelve randomized controlled trials that included 1237 patients revealed that iNO significantly improves oxygenation during the first days of inhalation, although no benefit on survival could be detected. Treatment with iNO was further burdened with an increased risk of renal dysfunction.8
The significant improvements in oxygenation and reduction of elevated PAP by iNO, however, could not be denied, and prompted scientists to study the effect of iNO in other diseases associated with severe hypoxia or pulmonary hypertension. Table 61-1 presents a literature survey, focusing on iNO therapy in several diseases or conditions in adults or older children. It further provides expert recommendations for the application of iNO in the respective patient collectives. Synopsis of the information compiled in the table allows for the conclusion, that in most cases of severe oxygenation impairment or high PAP, iNO is effective. When considering iNO as a possible therapeutic option in adult patients, it is important to recognize that certain pathophysiologic variables may significantly improve, although in no instance was survival clearly enhanced.
| Diagnosis | Effect of NO Inhalation | Expert Recommendations |
|---|---|---|
| Heart disease, heart failure (iNO for diagnostic purposes) | Pulmonary vascular reactivity testing with O2 and iNO can identify patients with pulmonary hypertension suitable for corrective cardiac surgery or heart transplantation.52 Patients with severe heart failure may experience pulmonary edema with inhalation of NO, probably because of increased left atrial filling after pulmonary vasodilation.53 | iNO trial for identification of patients suitable for cardiac surgery or heart/lung transplantation is recommended. iNO testing in patients with left-heart dysfunction is dangerous, heart function should be optimized before iNO testing.20 |
| Pulmonary arterial hypertension (iNO for diagnostic purposes) | iNO decreases PAP effectively in some patients. The acute decrease of PAP with iNO is the best predictor of long-term response to oral vasodilator treatment.54 iNO can identify responders for a long-term treatment with calcium-channel blockers.55 | iNO is recommended for acute vasodilator testing in a dosage of 10 to 20 ppm.20 Insufficient data to recommend long-term inhalation of NO.20 |
| Thromboembolism | In four cases of pulmonary embolism, iNO led to improvement of pulmonary hemodynamics and oxygenation.56 In four further patients with acute massive pulmonary embolism, inhaled NO rapidly improved pulmonary and systemic blood pressures, heart rate, and gas exchange.57 | No routine use of iNO in thromboembolic disease recommended because of insufficient data. In patients with severe right-ventricular failure or severe hypoxemia, iNO may be beneficial.20 |
| Sickle cell disease | Most common complications of sickle-cell disease are the vasoocclusive pain crisis and the acute chest syndrome, a form of lung injury, as well as hemolysis. Hemolytic anemia is associated with pulmonary hypertension.58 Results from two small randomized, placebo-controlled trials suggested that in severe vasoocclusive crisis, inhalation of 80 ppm NO decreased pain scores and morphine use.59,60 | No routine use of iNO recommended because of insufficient data.20 |
| Chronic obstructive pulmonary disease (COPD) | Effects of iNO in patients with COPD are contradictory. In one study, iNO improved oxygenation and reduced PAP while right-ventricular ejection fraction increased.61 In another study, iNO reduced PAP but did not improve right-ventricular ejection fraction or arterial oxygenation in patients with acute respiratory failure caused by acute exacerbation.62 In a third study, however, addition of iNO to inhaled oxygen did not improve or worsen arterial partial oxygen pressure, but caused a significant decrease in mean PAP. Cardiac output increased. Long-term use of iNO was effective.63 In ten volunteers with very severe COPD iNO in concentrations of 40 to 40,000 ppb did not improve oxygenation.64 | No evidence of a clinical benefit of iNO in patients with COPD.20 |
| High-altitude pulmonary edema (HAPE) | In mountaineers prone to HAPE, iNO produced a marked decrease in PAP. In subjects with radiographic evidence of pulmonary edema, iNO improved oxygenation.65 In fourteen patients with severe HAPE, iNO reduced PVR by 36% compared with room air. PVR fell by 54% when iNO was combined with 50% oxygen. 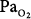 increased by 14% when iNO was applied.66 In a rat model of HAPE, iNO improved survival.67 increased by 14% when iNO was applied.66 In a rat model of HAPE, iNO improved survival.67 | No recommendation because of insufficient data. |
| Myocardial infarction and cardiogenic shock | In thirteen patients with right ventricular myocardial infarction and cardiogenic shock iNO significantly decreased PAP and PVR, and increased CI by 24%.68 Application of iNO before and during coronary reperfusion is able to reduce infarct size (animal experiment).69 | No recommendation because of insufficient data. |
| Right-ventricular failure (RVF) | RVF responds favorably to afterload reduction. In RVF after myocardial contusion or after right-ventricular myocardial infarction with cardiogenic shock, iNO rapidly improved hemodynamics.68,70 | No recommendation because of insufficient data. |
| Perioperative pulmonary hypertension and RVF in cardiac surgery | Cardiopulmonary bypass reduces NO production in pulmonary tissue that leads to pulmonary vasoconstriction and right-ventricular dysfunction postoperatively (animal experiment).71 Inhalation of NO during and after cardiopulmonary bypass diminishes the release of markers of myocardial injury. Left ventricular dysfunction during and immediately after cardiopulmonary bypass is antagonized.72 In twenty-three patients with pulmonary hypertension, treated with iNO immediately after cardiopulmonary bypass, iNO significantly reduced PAP and PVR and lead to increased cardiac output.73 | In patients with perioperative acute right-ventricular dysfunction and pulmonary hypertension, RVF should be optimized first by conventional measures before a trial of iNO should be undertaken. iNO doses >20 ppm have no advantage.20 |
| Left-ventricular assist devices (LVADs) | Pulmonary hypertension is frequent in patients treated with LVADs and may lead to right-ventricular dysfunction. Eleven patients with LVADs and pulmonary hypertension were randomized to iNO (20 ppm) and control therapy. iNO precipitated significant reductions in PAP and increased LVAD flow. In control patients, no hemodynamic improvement was recorded.74 A recent randomized controlled trial studied the effect of iNO in forty-seven patients with implanted LVADs. Only in the iNO group did PVR decrease significantly, from 311 ± 35 to 225 ± 17 dyn sec cm-5 (p < 0.01).75 | Expert panel believes that iNO improves pulmonary hemodynamics in patients with inadequate left-sided flow during use of LVADs and pulmonary hypertension refractory to conventional maneuvers. In this situation, they recommend application of iNO among other vasodilator therapies.20 |
| Heart transplantation | Pulmonary hypertension is a frequent problem during heart transplantation and may contribute to life-threatening right-heart failure. In heart transplant recipients with pulmonary hypertension, iNO given in the postoperative period selectively reduced PVR and enhanced right-ventricular stroke work. Compared with a historical cohort, the NO treated group had better survival rates.76 Fourteen patients with either heart transplantation or lung transplantation received iNO in the operating room when pulmonary hypertension, refractory hypoxemia, or right-ventricular dysfunction were present. Inhalation of 20 ppm NO lowered PAP and central venous pressure, increased cardiac index, and improved mixed venous oxygen saturation.77 | iNO is used by several institutions with experience in cardiac transplantation. They recommend it as standard therapy for heart transplantation complicated by an elevated PVR.20 |
| One-lung ventilation (OLV) | Hypoxia is a frequent complication during OLV. Sixteen patients who developed hypoxemia during OLV were randomized to iNO (20 ppm) or control groups (nitrogen). iNO when administered to the dependent lung was not superior to nitrogen.78 In patients with pulmonary hypertension during OLV, iNO caused a significant reduction of mean PAP and improved oxygenation in patients with severe hypoxemia.79 A combination of iNO with almitrine significantly improved oxygenation during OLV.26 | Expert panel does not recommend routine use of iNO during OLV. Only in case of severe hypoxemia, refractory to conventional therapy, iNO may beneficial.20 |
| Major lung resection | Postpneumonectomy pulmonary edema is a severe complication of lung resection and afflicted with high mortality rates. In a patient with major lung resection iNO was successfully applied to treat postpneumectomy pulmonary edema (case report).80 | No recommendation because of lack of data. |
| Lung transplantation | There remains controversy whether iNO can prevent ischemia–reperfusion injury in lung-transplant recipients. Some studies performed in the 1990s reported that iNO reduced reperfusion injury in transplanted lungs. Another study, however, showed that prophylactic iNO does not prevent reperfusion injury. During reperfusion, however, patients with reperfusion injury experienced improved oxygenation and reduction of PAP.81 Contrary to earlier studies, a large randomized, controlled trial could also not detect a significant effect on physiologic variables or outcomes in the group of iNO-treated patients, in whom iNO was initiated 10 minutes after reperfusion.82 The occurrence of acute graft rejection, however, was less frequent in the iNO group in comparison with historical controls.83 Recent randomized clinical trials could not detect a benefit of a prophylactic administration of iNO for prevention of primary graft failure, however, in case of development of a hypoxic phase during primary graft failure, iNO may reduce the need for extracorporeal membrane oxygenation in lung transplant patients.84 | No evidence that iNO prevents reperfusion injury after lung transplantation.20 |
| Acute lung injury (ALI)/ acute respiratory distress syndrome (ARDS) | iNO was found to reduce elevated PAP, improve oxygenation, and decrease intrapulmonary right-to-left shunt during the first days of treatment.31 A meta-analysis of twelve randomized controlled trials that included 1237 patients revealed that iNO improves oxygenation during the first days of inhalation. No benefit on survival could be detected. Risk of renal dysfunction was increased in the iNO patients.8 These findings were confirmed by a large systematic review of the Cochrane Collaboration.29 | No routine use of iNO in ALI/ARDS. Trial of iNO as a rescue treatment in case of life-threatening hypoxemia.20 |
| Influenza | Patients with H1N1 influenza very rapidly develop respiratory failure. Thirty-two patients of the H1N1 influenza epidemic 2009 in Spain were evaluated and 25% received iNO as a rescue therapy.85 In Canada, 168 critically ill patients with influenza A (H1N1) were admitted to thirty-eight intensive care units and prospectively evaluated; 13.7% of the patients received iNO as a rescue therapy.86 | ICUs treating influenza patients should provide advanced ventilatory support and rescue therapies including iNO.87 |
| Inhalation injury | There are only few reports on use of iNO in burn patients with inhalation injury.88–90 All studies reported an improved oxygenation with iNO therapy. An immediate and stronger early response to NO inhalation may eventually predict recovery, however, all studies were only performed in a small series of patients with the respective weakness of the reported results. | No routine use of iNO in inhalation injury. Trial of iNO as a rescue treatment in case of life-threatening hypoxemia after conventional treatment has been optimized. In case of early response to iNO, the inhalation should be continued.91 |
| Asthma | Inhalation of NO results only in a minor relaxation of airway tone in adults.92,93 In children with asthma, iNO has no apparent bronchodilatory effect.94 In case of severe status asthmaticus, however, iNO might have a bronchodilatory effect (case report).95 In five children with life-threatening status asthmaticus who required MV and did not respond to maximal medical management, iNO decreased 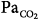 significantly. Four children survived.96 significantly. Four children survived.96 | No recommendation as to date no controlled studies on this topic have been performed. |
What are still suitable indications for iNO in adults or older children? It may be indicated in patients who are in a phase of severely impaired gas exchange that is unresponsive to maximal medical therapy. In such settings, application of iNO can significantly enhance pulmonary gas exchange and thereby prevent hypoxic organ damage. Moreover, iNO can be tried in all patients suffering from severe pulmonary hypertension. In most cases, elevated PAP drops and relieves the right heart. When considering possible iNO treatment, however, it is strongly recommended to optimize conventional treatment before a trial of iNO is undertaken.
Application of iNO yielded the best results in critically ill infants. In 1999, the use of iNO was approved by the United States Food and Drug Administration (FDA) for the treatment of term and near-term newborns (>34 weeks of gestation) with hypoxic respiratory failure associated with pulmonary hypertension.
In neonates with respiratory failure, persistent pulmonary hypertension of the newborn (PPHN) is common. PPHN is characterized by elevated pulmonary resistance, pulmonary vasoconstriction, and altered vascular reactivity. Desaturated blood circulates partly through an extrapulmonary right-to-left shunt, across the foramen ovale and ductus arteriosus. Since 1970, extracorporeal membrane oxygenation (ECMO) has been the treatment of choice if PPHN is present. Survival rates of up to 80%9 were achieved with ECMO therapy whereas survival with conservative therapy was approximately 50%.10 In hypoxemic newborns with PPHN, clinical studies indicate that iNO increases systemic O2 levels, decreases PAP, and mitigates ventilation–perfusion mismatch. Randomized, placebo-controlled trials of iNO in neonates with PPHN failed to show a significant decrease in mortality rates in the iNO group; iNO therapy did, however, reduce the requirement for ECMO.11,12
Some years ago, a large, randomized, controlled trial documented the effectiveness of iNO in 207 preterm neonates with respiratory failure. iNO decreased the incidence of chronic lung disease and death.13 These encouraging findings, however, were not supported by further studies. A recent systematic review of fourteen randomized controlled trials that studied iNO therapy in preterm neonates unveiled a disappointing picture. iNO was not able to significantly decrease mortality rates and early rescue treatment is probably associated with a higher risk of intraventricular hemorrhage.14 In a selected patient collective of premature infants with a birth weight between 1000 and 1250 g, however, early inhalation of NO might reduce the incidence of bronchopulmonary dysplasia.15
Perioperative pulmonary hypertension in infants with congenital heart defects is harmful and compromises their chance of survival. iNO, however, has not proven to reduce mortality or the number of pulmonary hypertensive crises.16 A trial with iNO could only be recommended in infants with severe pulmonary hypertension in the perioperative setting of congenital heart surgery.17,18
From a pathophysiologic point of view, iNO therapy may be harmful for newborns with congenital heart disease dependent on right-to-left shunts and its routine use is not recommended in this setting.11 Newborns with congestive heart failure and lethal congenital anomalies should also be excluded from iNO therapy.11











