2. Fetal lung development takes place throughout pregnancy, and lung maturation continues well into the neonatal period. The pulmonary transition that occurs in the fetus is initiated before labor begins. Cortisol production increases in the third trimester to begin multisystem organ preparation for the newborn transition, including preparation for pulmonary maturation. In addition, surfactant production increases to reduce alveolar surface tension, promote alveolar expansion, foster gas exchange, and stimulate fetal lung development.5
B. Normal peripartum transition to extrauterine life
1. Delivery leads to rapid and profound changes in neonatal hemodynamics and pulmonary mechanics. During development, the fetal airways contain approximately 30 mL per kg of fluid. This fluid, an ultrafiltrate of fetal plasma, begins to reabsorb during labor.3 During vaginal delivery, compression of the thorax expels further fluid from the mouth and upper airways.2 With the first breaths, the lungs fill with air, surfactant is released, and oxygenation increases dramatically.6 This increase in oxygen tension and blood flow increases the release of nitric oxide in the pulmonary vasculature resulting in pulmonary vasodilation and a substantial decrease in PVR.7 Simultaneously, clamping of the umbilical cord removes the low-resistance placenta from the systemic circulation increasing SVR. Functional closure of the foramen ovale occurs rapidly after birth as left atrial pressures exceed right atrial pressures.
2. The right-to-left shunt across the foramen ovale and ductus arteriosus is substantially reduced within the first few minutes of life.
CLINICAL PEARLThe substantial right-to-left shunting of oxygenated blood in the fetal circulation is dramatically reduced at birth by reductions in PVR and increases in SVR. These changes are primarily due to the neonate’s first breaths and umbilical cord clamping.
C. Prolonged hypoxemia/acidosis and failure of transition
1. Transient hypoxemia and acidosis are typically well tolerated in the normal newborn; prompt resuscitation of a depressed newborn should prevent permanent physiologic alterations. However, prolonged neonatal hypoxemia and acidosis impede the normal transition from fetal to neonatal physiology. Hypoxemia maintains patency of the ductus arteriosus and continued right-to-left shunting as ductal smooth muscle constriction depends on an increase in blood oxygen tension. Hypoxemia promotes hypoxic pulmonary vasoconstriction and increases the risk of significant pulmonary hypertension. Pulmonary hypertension causes an elevation of right atrial pressure, maintaining the right-to-left shunt across the foramen ovale. Blood flowing through the patent ductus arteriosus and foramen ovale is therefore not oxygenated, contributing to increasing hypoxemia and continued deterioration.4
2. Both the fetus and neonate respond to hypoxemia with a “diving” reflex (known as such due to its similarity to seal physiology during a dive): Blood flow is diverted centrally to the heart, brain, and adrenal glands, and tissue oxygen extraction increases.
3. Neonatal circulation initially exhibits a hypertensive response because additional oxygen cannot be extracted by the neonate. As a result, myocardial contractility and cardiac output decrease leading to systemic hypotension.4
4. Neonatal ventilation during hypoxia is initially rapid and regular (see Fig. 17.2). However, as hypoxia continues, respirations stop in a stage known as primary apnea. Stimulation of the neonate during primary apnea will lead to reinitiation of respiratory effort. If hypoxia continues through primary apnea, the neonate will begin to attempt irregular, grunting respirations, followed by secondary or terminal apnea. Stimulation will not reverse secondary apnea because respiratory drive has been reduced by both central nervous system and direct diaphragmatic depression.8 The net result of these physiologic responses is a neonate with persistent pulmonary hypertension and little or no ventilatory drive. Ideally, prompt resuscitation prevents these physiologic perturbations.
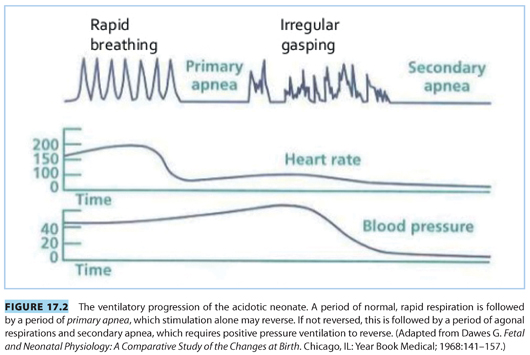
CLINICAL PEARLTransient hypoxemia and acidosis are well tolerated by the normal newborn. However, prolonged hypoxemia and acidosis lead to depressed hemodynamics, persistent pulmonary hypertension, and poor ventilatory drive.
II.Anticipating the depressed newborn
Neonatal resuscitation can be anticipated in about 80% of cases.1,9
A. Antepartum evaluation (see Chapter 7 Fetal Assessment and Monitoring)
The advent of fetal heart rate (FHR) evaluation by cardiotocography has allowed for development of protocols to identify the at-risk fetus and, if necessary, effect delivery and outcome. The nonstress test (NST), contraction stress test (CST), and the biophysical profile (BPP) are all used to assess fetal well-being10–15 (see Chapter 7). Many of the antepartum factors related to increased likelihood for neonatal resuscitation are listed in Table 17.1.
B. Intrapartum evaluation (see Chapter 7)
Many intrapartum events increase the likelihood for neonatal resuscitation (see Table 17.2).
1. The primary method for intrapartum evaluation of the fetus is FHR monitoring by cardiotocography. Although this is a reliable method of confirming fetal well-being and is highly predictive of the need for immediate postpartum resuscitative efforts,9,16 a reassuring FHR pattern does not necessarily guarantee a neonate who does not need resuscitation at birth; 50% of babies born by cesarean delivery (CD) with prior reassuring FHR patterns will require some resuscitation.9
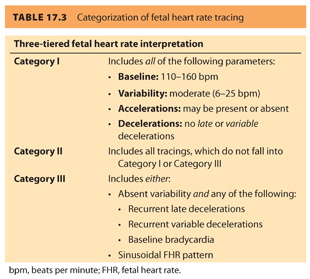
2. Intrapartum fetal monitoring utilizes FHR measurement (by external Doppler ultrasound or internal fetal scalp electrode) combined with measurement of contraction pattern (by external tocodynamometry or intrauterine pressure transducer). These two parameters together can suggest fetal well-being or foretell fetal asphyxia. Four parameters are evaluated when assessing the FHR tracing: baseline, variability, accelerations, and decelerations.17 Using these four parameters, FHR tracings can be placed into three categories, as identified in Table 17.3. Category I tracings are classified as normal and are indicative of fetal well-being. Category II tracings are classified as indeterminate and are neither indicative of fetal well-being nor predictive of abnormal fetal acid–base status. Category III tracings are abnormal and are highly predictive of abnormal fetal acid–base status and should be met with prompt evaluation and intervention.17 In the past, fetal scalp sampling has also been used to help confirm the need for expedited delivery (indicated if the fetal capillary pH was below 7.20).18 However, the examination is invasive and has been replaced by fetal scalp stimulation. An FHR acceleration of 10 beats per minute (bpm) for 10 seconds or longer after stimulation is highly predictive of a fetal pH greater than 7.20.19
CLINICAL PEARLA majority of neonates who will require resuscitation can be anticipated by ante-and intrapartum fetal assessment; however, resources should be available to resuscitate a neonate at every delivery, if required.
III.Evaluating the neonate
A. Apgar score. Historically, the Apgar score was the first standardized approach to neonatal evaluation following delivery. The score, first published by anesthesiologist Virginia Apgar in 1953, uses five signs: heart rate, respiratory effort, reflex irritability, muscle tone, and color, each on a 0-to-2-point scale, for a maximum score of 10, measured at 1 and 5 minutes postpartum.20 The individual parameters are listed in Table 17.4. The Apgar score contains the major elements used in more current algorithms to guide therapy of the depressed neonate following delivery.21
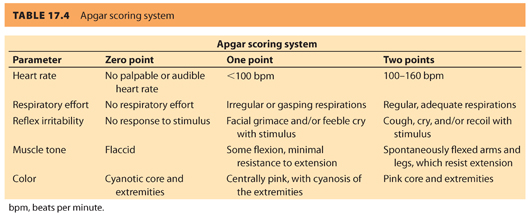
1. In addition, the Apgar score has repeatedly proven useful in predicting neonatal mortality; the term infant with a 5-minute Apgar score of 0 to 3 is more likely to die in the neonatal period than the term infant with a score of 7 to 10.21,22
2. Use of the Apgar score is not without concerns. There is high interobserver variation in scoring, both in the total score and in each of the subcategories.23 In addition, the scores have been used incorrectly in an attempt to identify long-term outcomes beyond the increased risk of neonatal death (including long-term neurologic function, cerebral palsy, and even future intelligence). This has led to concerns of increased medicolegal liability based on a scoring system that, although shown to correlate with neonatal death, is not “a conclusive marker . . . of an acute intrapartum hypoxic event” according to the American Academy of Pediatrics (AAP) and the American College of Obstetricians and Gynecologists (ACOG).24
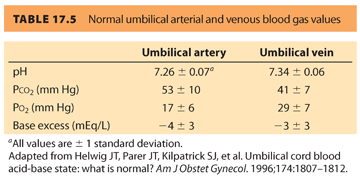
B. Umbilical cord blood gas measurements. Umbilical cord blood gas measurements can be used to evaluate the intrauterine environment of the fetus prior to delivery. Normal umbilical arterial and venous blood gas measurements are found in Table 17.5.
CLINICAL PEARLElements of the Apgar score can be used to guide resuscitation of the neonate following delivery.
IV.Resuscitation of the neonate
All anesthesia providers working within labor and delivery units must be familiar with neonatal resuscitation. Given the urgent nature of neonatal resuscitation, preparation is imperative.
A. Preparation for resuscitation
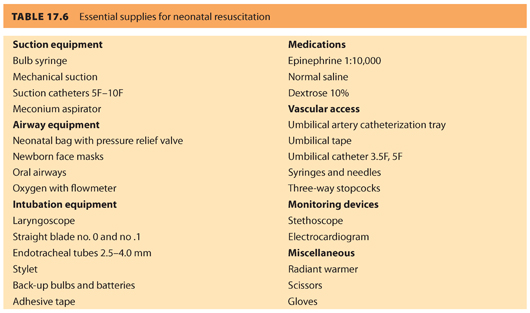
Neonatal resuscitation equipment and medications should be organized in a central location, checked frequently for proper function and expiration, and replenished immediately after use (see Table 17.6). At least one provider whose primary responsibility is resuscitation of the newborn should attend every delivery. Both the American Society of Anesthesiologists (ASA) and the ACOG have published optimal goals for neonatal resuscitation endorsing that the anesthesiologist’s primary responsibility is care of the mother.25 However, the anesthesiologist may provide brief neonatal assistance only if care of the mother is not compromised. In addition, the joint statement recommends that individuals demonstrate proficiency in evaluating the neonatal condition, knowledge of the pathophysiology of neonatal depression, and skill in airway management and drug administration. Additional qualified personnel are recommended for deliveries where fetal compromise is anticipated.
CLINICAL PEARLThe primary responsibility of the anesthesiologist is to care for the mother. A second individual skilled in neonatal resuscitation should be present, if possible, when neonatal resuscitation is anticipated or needed.
B. The resuscitation algorithm
The American Heart Association (AHA) and AAP endorse a neonatal resuscitation protocol based on frequent assessment of the newborn and escalating levels of intervention (see Fig. 17.3).1 The algorithm is organized into 30-second intervals during which an intervention is completed, the neonate is reassessed, and the decision to proceed, or not, is made. Assessment of the neonate focuses on evaluation of the heart rate, respirations, and oxygenation. Successful completion of the previous step is a prerequisite to proceeding to the next intervention.
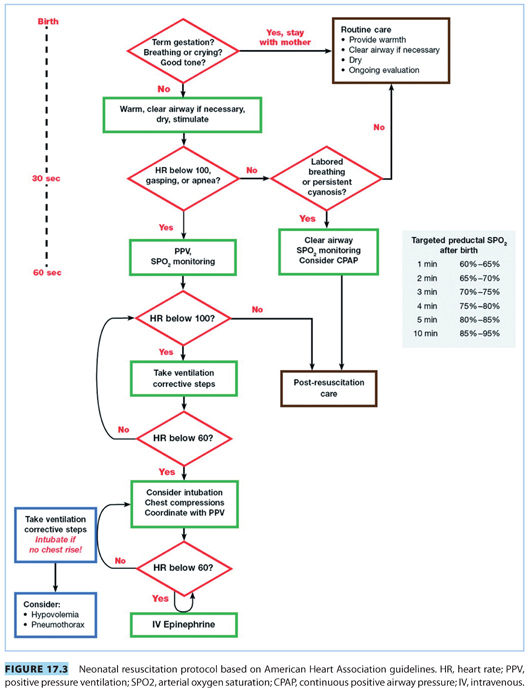
C. Initial resuscitation
The general condition of the neonate is quickly assessed with four screening questions addressed immediately following delivery (see Fig. 17.3): (1) Is the infant full-term? (2) Is the amniotic fluid clear? (3) Is the newborn breathing or crying? (4) Does the infant have good muscle tone? Routine care is provided for full-term newborns with clear amniotic fluid, good muscle tone, and breathing without distress. Neonates with difficulty making the transition to extrauterine life should be warmed, gently stimulated, and placed in the “sniffing position” to open their airways. Suctioning of the pharynx and nose should be brief and gentle because prolonged or vigorous suctioning may result in breath holding, laryngospasm, or bradycardia.
D. Maintain normothermia
Minimizing heat loss is an integral part of neonatal resuscitation. All infants have an unstable thermal regulatory system and this is enhanced in depressed or asphyxiated neonates. The large surface area to body mass ratio of the neonate facilitates rapid heat loss by conduction, convection, evaporation, and radiation. The term neonate’s primary defense against hypothermia is nonshivering thermogenesis through catecholamine-mediated metabolism of brown fat. This, in turn, leads to an increase in oxygen consumption, calorie utilization, and metabolic rate.26 The resulting hypoxemia, hypercarbia, and hypoglycemia promote persistence of the fetal circulation and hinder resuscitation.
E. Assisted ventilation
Spontaneously breathing infants should make their first respiratory effort seconds after delivery of the thorax, generating a negative intrathoracic pressure of 60 to 100 cm H2O and inspiring a tidal volume of approximately 80 mL. The majority of this first breath is retained within the lungs as part of the functional residual capacity.27 Infants who fail to initiate respirations with gentle stimulation, warming, and airway suctioning require further intervention to ensure adequate ventilation.
1. Assisted ventilation is required in about 3% to 5% of all newborn infants. Positive pressure ventilation is indicated in neonates who remain apneic, have ineffective or gasping ventilation, or a heart rate less than 100 bpm more than 30 seconds after delivery (see Fig. 17.3). Assisted ventilation can be initiated via a bag valve mask device, a laryngeal mask airway (LMA), or an endotracheal tube. Ventilation is usually first established with a bag valve mask device followed by endotracheal intubation in the absence of clinical improvement.
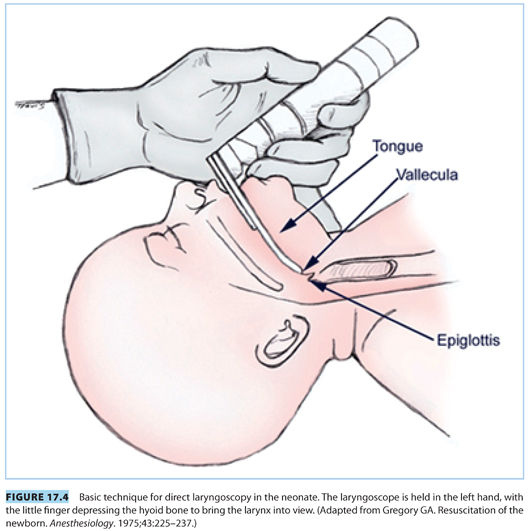
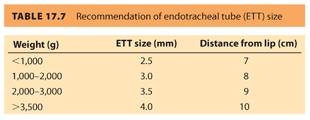
2. Common indications for endotracheal intubation include tracheal suctioning for meconium, ineffective or prolonged bag valve mask ventilation, or endotracheal administration of medications (although this practice is now strongly discouraged). For ideal intubating conditions, the infant’s head is placed in a neutral “sniffing position” (see Fig. 17.4). A small straight blade, such as a Miller 0 or 1, provides the best visualization of the neonatal larynx given its unique anatomic characteristics. Specifically, the newborn larynx is more anterior than the adult and at the level of the third cervical vertebra rather than the sixth. A small air leak with positive pressure ventilation indicates the uncuffed endotracheal tube is appropriately sized (see Table 17.7). An oversized endotracheal tube may cause subglottic stenosis, whereas an undersized endotracheal tube can impede adequate ventilation and may become easily occluded. The endotracheal tube is inserted 2 cm past the vocal cords, and tracheal placement is confirmed via detection of end tidal CO2, bilateral breath sounds, and symmetric chest rise.
3. Both bag valve mask and endotracheal ventilation are known to produce significant morbidity. Prolonged bag valve mask ventilation can result in distention of the stomach and/or ocular or facial abrasions from pressure applied to the mask. On the other hand, direct laryngoscopy can be accompanied by a significant hypertensive response contributing to cerebral hemorrhage. In addition, intubation can be challenging in neonates. A recent survey found that intubation by third-year pediatric residents was successful only 62% of the time on the first or second attempt.28
4. Given some of the limitations of bag valve mask and endotracheal ventilation, some advocate for the use of LMAs in neonatal resuscitation.29–31 LMAs have been used successfully in resuscitation of both full-term and preterm infants and may have significant advantages over endotracheal intubation, including ease of use and decreased hemodynamic response. Both novice and experienced providers have had success with LMA use in the neonate. One study reported the successful resuscitation of 20 neonates with the LMA as a conduit for positive pressure ventilation by novice providers.29 Another trial found no significant difference between the LMA and endotracheal intubation during resuscitation of infants by experienced providers after CD.31 Although endotracheal intubation is still preferred in situations requiring ventilation with high peak airway pressures, tracheal suctioning, or administration of endotracheal medications, the LMA can be lifesaving in neonates with difficult airways, specifically in conditions with a congenital hypoplastic mandible, such as Pierre-Robin and Treacher-Collins syndromes.
CLINICAL PEARLNeonatal respiratory depression at birth will often respond to stimulation, warming, and gentle suctioning. Assisted ventilation with bag and mask should be performed in those who do not respond to those interventions.
F. Considerations for establishing ventilation
The optimal inflation pressure, time, and flow rate to establish ventilation in the neonate have yet to be determined. With assisted ventilation, pressures of 30 to 40 cm H2O are often necessary for the first few breaths to establish lung expansion with the use of higher inflation pressures carrying the risk of iatrogenic pneumothorax. After initial inflation, pressures of 12 to 20 cm H2O are usually sufficient to deliver tidal volumes of 5 to 7 mL per kg. If positive pressure ventilation fails to initiate spontaneous respiration, ventilation should be continued at a rate of 30 breaths per minute. Consideration should be given to the application of positive end-expiratory pressure (PEEP) because it may protect against lung injury and improve lung compliance and gas exchange.32 Regardless of the mode of ventilation, adequate oxygenation is confirmed by an improvement in heart rate, color, and body tone.
G. Oxygen administration
The administration of oxygen has historically been a fundamental aspect of neonatal resuscitation; however, recent evidence challenges the use of 100% oxygen in neonatal resuscitation.33–37 In fact, in the most recent guidelines, the AHA and AAP support the use of room air during initial resuscitative efforts for the full-term infant.1
1. The growing interest in room air resuscitation began with a report by Ramji et al.33 that room air was as effective as 100% oxygen in neonatal resuscitation. Follow-up investigations reported a decreased neonatal mortality rate (13.9% vs. 19%) in infants resuscitated with room air instead of 100% oxygen,34 although no significant differences in neurologic evaluation, somatic growth, or developmental milestones at 18 to 24 months were found between the two groups of infants.35 A recent report from Spain demonstrated a reduction in mortality from 3.5% in the 100% oxygen group to 0.5% in the room air group, suggesting the reduction in mortality is not exclusive in studies conducted in third world environments.37 The benefits of room air neonatal resuscitation, including a significantly lower neonatal mortality, shorter time to first breath, and higher 5-minute Apgar scores in the room air group, were recently summarized in a meta-analysis encompassing randomized or pseudo-randomized trials of neonatal resuscitation with room air versus 100% oxygen.36
2. Despite growing evidence that resuscitation with 100% oxygen may be harmful, it remains unclear that ventilation with room air is ideal; further studies of the effect of inspired oxygen tension on neonates after resuscitation have reported differing results.38–42 In a neonatal piglet model of intermittent apnea, selective regions of the brain (striatum and hippocampus) were better protected from apoptotic injury when resuscitation was conducted with 100% rather than 21% oxygen.38 Other studies have suggested that resuscitation with 100% oxygen leads to reperfusion injury in the developing brain, lungs, myocardium, and kidneys secondary to excessive production of reactive oxygen species.39–41 In addition, exposure to even brief periods of hyperoxia following delivery has been associated with decreases in cerebral blood flow in term and preterm infants.42
3. It remains unclear what oxygen fraction is optimal to achieve a balance between the toxic effects of oxygen and the deleterious effects of hypoxemia.
CLINICAL PEARLAlthough the use of 100% inspired oxygen during resuscitation has been recently challenged, the optimal inspired oxygen fraction has not yet been determined.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







