2. Despite the very low incidence of neurologic injury, it represents a significant contributor to malpractice litigation. Davies et al.11 performed an obstetric anesthesia closed claims analysis of 426 claims from 1990 to 2003. Regional anesthesia was involved in nearly 80% of the claims, whereas general anesthesia was involved in 17%. Headaches accounted for 10% of the claims, back pain 8%, and nerve injury 20%. Nerve injury payments averaged $126,000. Lee et al.9 performed a closed claims analysis of 1,005 regional anesthesia claims from 1990 to 2004. Cardiac arrest associated with neuraxial block was the primary damaging event in 32% of obstetric claims involving death or brain damage. Although, obstetric anesthesia claims are predominately associated with minor injuries, cardiac arrest associated with neuraxial anesthesia and neuraxial hematomas associated with coagulopathy are the main sources of high-severity injuries.
3. Hayes et al.12 collected self-reported patient symptoms following discharge. At this single institution in Ireland from 2004 to 2007, 15,033 deliveries occurred with 46.5% receiving neuraxial anesthesia. Only 1.4% (98) patients contacted the investigators with new complaints. Of these, headache was the most common complaint (44% of complaints), presenting 5 to 9 days (interquartile range) postpartum with only 4 of the 43 patients receiving an epidural blood patch (EBP). Sensorimotor symptoms were self-reported in 34% of patients who self-reported, with a median time reporting on day 8 postpartum. The incidence of late, self-reported obstetric nerve palsies was 1:15,033.
4. Horlocker et al.13 reported their experience with 4,220 epidurals inserted for postoperative analgesia in nonobstetric patients. These patients were anesthetized before insertion of the neuraxial catheter and therefore could not report paresthesias. One epidural catheter broke during removal and a segment was retained; there were no other major problems. Six patients developed new neurologic symptoms or postoperative worsening of a previous neurologic condition unrelated to epidural catheterization. There was one death due to anterior spinal artery syndrome. The patient had a prolonged aortic cross-clamp time, which might have predisposed to spinal cord ischemia. The authors concluded that the risk of neurologic complications associated with lumbar epidural catheter placement in anesthetized patients is small.
5. Wong et al.21 studied 6,057 women who delivered liveborn infants; 6,048 were interviewed and 56 had a confirmed new nerve injury to a lower extremity peripheral nerve, an incidence of 0.92%. Factors found by logistic regression analysis to be associated with nerve injury were nulliparity and prolonged second stage of labor. Women with nerve injury spent more time pushing in the semi-Fowler–lithotomy position compared to women without injury. The median duration of symptoms was 2 months. Contrary to the previously described studies, this study reports a much higher incidence of neurologic injury. However, the injuries reported in this study seem to be related to childbirth and not due to the anesthetic technique per se.
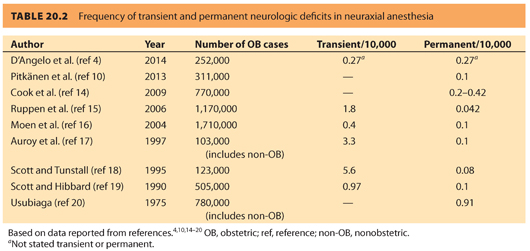
6. A review by Brull et al.,22 of 32 studies published over 10 years, suggests that the rate of neurologic complications after central nerve blockade is <4:10,000 or 0.04%. This review also suggests that spinal anesthesia carries a higher risk of radiculopathy or peripheral neuropathy (3.78:10,000) compared to epidural anesthesia (2.19:10,000) (see Fig. 20.1). However, permanent neurologic injury after neuraxial anesthesia is a rare occurrence in contemporary anesthetic practice.
CLINICAL PEARLNeurologic injury following neuraxial blockade in obstetrics is very rare but contributes to 80% of patient claims for injury associated with obstetric anesthesia.
II.History and initial evaluation
When evaluating a patient with postpartum neuropathy, it is important to remember that the etiology of neuropathy may be totally unrelated to anesthesia and may be secondary to nerve injury sustained by the patient during the birth process. Nerve injury may occur during vaginal birth or cesarean delivery (CD); however, mode of delivery does not seem to carry a different risk of neurologic injury.3
A. Relevant questions during evaluation. The most pertinent questions to ask when evaluating a patient with postpartum neuropathy are6–8:
1. What was the duration of labor?
2. How long did the patient push?
3. Was the patient placed in exaggerated lithotomy position while pushing?
4. Were forceps or vacuum used?
5. What was the weight of the infant?
6. What was the position of the presenting part (i.e., occiput posterior)?
7. Did the patient have any history of back problems or preexisting neurologic disorder (e.g., multiple sclerosis [MS], HIV infection, diabetes, obesity)?
8. What was the type and amount of local anesthetic used?
9. Did the patient ever recover sensory and motor function completely before the onset of symptoms?
10. What type of anesthetic was delivered and its duration?
B. The causes of postpartum neurologic injury
Neuraxial anesthesia is only one of many possible etiologies of neurologic injury.23 These injuries are often the result of direct trauma caused by the fetal head, or obstetric forceps, to the major nerve trunks that supply the lower extremities. Direct ischemic injury of the lower spinal cord is also possible when the fetal head compresses the ascending spinal branch of the internal iliac artery.8 One should also consider the possibility of epidural hematoma. When extensive neurologic deficits are noted, a neurologist or neurosurgeon must be consulted. Bilateral leg weakness may be due to spinal cord compression (e.g. epidural hematoma), and magnetic resonance imaging (MRI) or computed tomography (CT) scan of the spinal cord must be performed without delay. Neurologic deficits due to spinal cord compression may be reversible if diagnosed early, and decompression occurs within the first 6 to 12 hours.
C. The extent of the injury
Neurologic injuries associated with childbirth may involve many disparate areas, including the lumbosacral plexus, anterior tibial nerve, femoral nerve, obturator nerve, and lateral femoral cutaneous nerve, and may rarely result in cauda equina syndrome.6–8 Obviously, involvement of a major nerve plexus may result in extensive injury that can take weeks or months to resolve. Rarely, piriformis syndrome, an inflammation of muscle fascia affecting the sciatic nerve, may occur.24,25 The basic anatomy of neural and vascular structures of the pelvis will be reviewed because injury to these structures can result in serious neurologic complications.26
III.Basic anatomy
A. The lumbar plexus
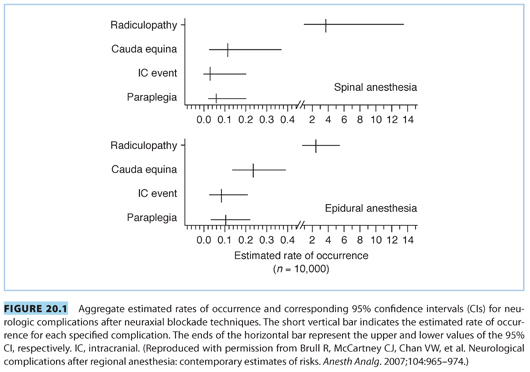
1. The lumbar plexus and its branches (see Fig. 20.2) may be compressed by the fetal head. The lumbar plexus is formed by the communication of the upper four lumbar nerves.27 The lumbar plexus is connected to the sacral plexus by the lumbosacral trunk. The plexus assembles in the substance of the psoas major muscle, anterior to the transverse processes of the lumbar vertebrae.
2. The branches of the lumbar plexus include the iliohypogastric, ilioinguinal, genitofemoral, lateral cutaneous, femoral, and obturator nerves, and branches to the psoas major and minor, iliacus, and quadratus lumborum muscles.
B. The sacral plexus
The sacral plexus (see Fig. 20.2) is formed by the contribution of nerves from L4, L5, S1–S3, and a part of S4. The coccygeal plexus receives the remainder of the S4 and S5 nerve roots and the coccygeal nerve. The roots of the lumbosacral plexus unite to form two major divisions, the sciatic and the pudendal nerves. The plexus lies on the posterior wall of the pelvic cavity, behind the pelvic fascia, and on the anterior surface of the piriformis muscle.
C. Trauma caused by the fetal head
As the fetal head crosses the ala of the sacrum (posterior brim of the pelvis), it can compress the lumbosacral plexus (see Fig. 20.2).8 This type of injury can be unilateral (75%) or bilateral (25%), and occurs more commonly in nulliparous parturients with a platypelloid pelvis, large fetus, cephalopelvic disproportion, vertex presentation, and forceps delivery.28,29 These compressive nerve injuries may involve multiple root levels and can present as injuries to the femoral or the obturator nerve, with sensory impairment of L4–L5 dermatomes. The incidence of obstetrical lumbosacral plexopathy occurs in 1.5 to 5/10,000 deliveries.3
IV.Common obstetric neuropathies
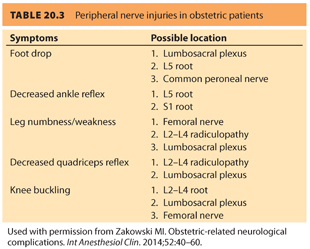
The anatomic features of some nerves make them especially vulnerable to damage during delivery. The distinguishing clinical picture of damage to these nerves is listed in Tables 20.3 and 20.4.
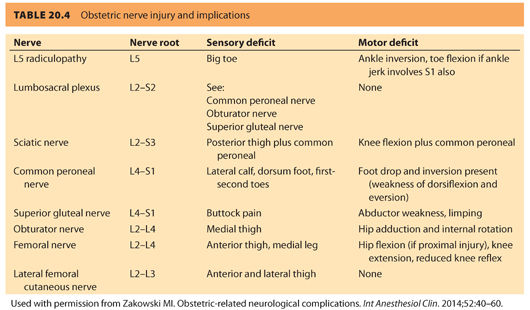
A. The lateral femoral cutaneous nerve of the thigh. This nerve (L2–L3) exits the pelvis 1 to 2 cm medial to the anterior superior iliac spine, under or through a split in the lateral end of the inguinal ligament. This nerve is purely sensory providing innervation to the anterior aspect of the thigh (see Tables 20.3 and 20.4 and Fig. 20.3). Relapses of MS may often affect this nerve.30 Decreased sensation of the anterior aspect of the thigh occurs as a result of compression of this nerve and is called meralgia paresthetica. In <2% of women, the lateral femoral cutaneous nerve arises from the femoral nerve and penetrates the inguinal ligament to exit the pelvis in which case, meralgia paresthetica is more likely to occur during pregnancy.31 The nerve is more likely to sustain trauma when the hyperextended lithotomy position is used. Pressure from the fetal head or from the handle of a retractor held by a surgical assistant during a CD may also lead to injury. In one prospective study, the lateral femoral cutaneous nerve was the most common obstetric nerve palsy, accounting for 30% of nerve injuries.5 This injury does not require treatment and typically recovers within 6 weeks.
B. The femoral nerve. The femoral nerve is composed of the nerve roots from L2, L3, and L4, exits beneath the inguinal ligament, and travels one fingerbreadth lateral to the femoral artery (see Fig. 20.2).31 The intermediate and medial cutaneous branches innervate the thigh, and it innervates the quadriceps femoris as well. The femoral nerve supplies the muscles and skin of the anterior compartment of the thigh, the psoas, and iliacus muscles. When this nerve is affected, hip flexion and knee extension become difficult. Because the hips are actively flexed during the second stage of labor, the inguinal ligament compresses the femoral nerve. Extreme or prolonged flexion of the hips during pushing should be avoided and the legs rested between contractions/pushing. The use of a squatting bar, to keep the hips hyperflexed during second stage of labor, has resulted in femoral nerve injury.6,7,31 Weak hip flexion suggests an injury to the femoral nerve at the level of the inguinal ligament. This may also occur due to compression of the lumbosacral plexus by the fetal head.
C. The obturator nerve. The obturator nerve (L2–L4)31 emerges at the medial border of the psoas at the pelvic brim. After passing through the obturator canal at the lateral wall of the pelvis (common locations of injury), it supplies the adductor muscles of the thigh along with the sciatic nerve. The adductor magnus muscle receives dual innervations by the obturator as well as by the sciatic nerve. When the obturator nerve is involved, thigh adduction is weakened with loss of sensation on the medial aspect of the thigh (see Tables 20.3 and 20.4 and Fig. 20.3).
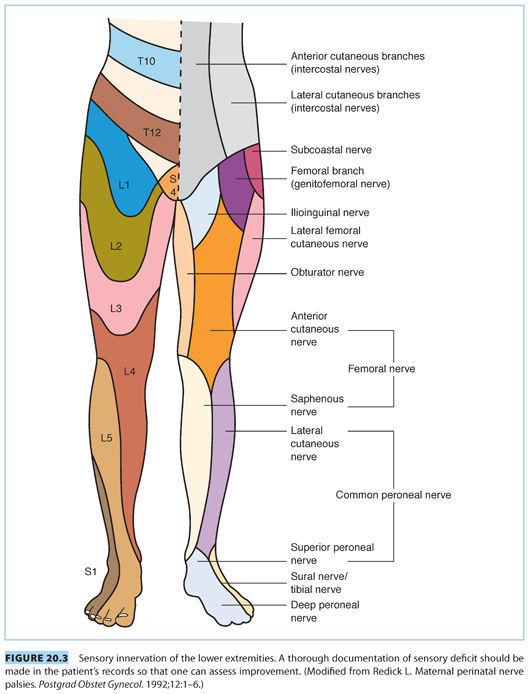
D. The sciatic nerve. The sciatic nerve (L4, L5, S1–S3)31 is the largest peripheral nerve in the body (see Tables 20.3 and 20.4, and Fig. 20.2 and 20.3). It leaves the pelvis through the greater sciatic foramen and divides into the larger tibial and smaller peroneal trunks. After exiting the sciatic foramen, it either exits inferior and through, or cranial to, the piriformis muscle, and then curves around the ischial spine and descends. The sciatic nerve innervates the adductor magnus and biceps femoris muscles and divides into medial (anterior tibial) and lateral popliteal (common peroneal) nerves in the popliteal triangle. The medial popliteal nerve supplies cutaneous (sural nerve) and muscular branches to the gastrocnemius and soleus muscles. The common peroneal nerve (L4, L5, S1, S2) winds around the neck of the fibula, where it is the only palpable nerve in the lower extremity (see Fig. 20.4). The nerve supplies both sensory and motor innervation to the leg. Owing to its superficial location, it is one of the more frequently injured nerves. Damage to this nerve causes paralysis of the ankle and foot, resulting in foot drop and inversion with sensory impairment of the anterior aspect of the foot (see Tables 20.3 and 20.4 and Fig. 20.3).32 Occasionally, inflammation of the piriformis muscle can lead to sciatic nerve irritation (piriformis syndrome) and cause gluteal and hip pain radiating down to the knee. Pain occurs when the extended thigh is rotated internally (Friedberg test).26,33 Prolonged sitting and excessive weight bearing during pregnancy, or injury during labor, can lead to irritation and spasm of the piriformis muscle. The peroneal portion of the sciatic nerve has a greater susceptibility to injury than the tibial division because the peroneal division travels more superficially in the bundle, has fewer and larger fascicles, less epineurium, and little blood supply. The peroneal nerve is fixed at two points, the sciatic foramen and the fibular head; sciatic neuropathy at the hip may appear similar to the more distal common peroneal nerve injury, with symptoms of foot drop and denervation of the anterolateral leg muscle. For suspected peroneal nerve injury, an MRI of the nerve at both the hip and knee should be performed.3 An MRI usually shows a hyperintense signal in the piriformis region.25,26 Electrophysiologic studies may be of assistance in diagnosis, but difficult to obtain given the deep location of the injury.
CLINICAL PEARLMost neurologic deficits can be diagnosed with a careful history and physical examination.
V.Ischemic injury to the spinal cord
A. Blood supply to the spinal cord. The blood supply to the spinal cord (see Fig. 20.5) can be precarious and subject to major anatomic variations.34–37 One anterior and two posterior spinal arteries supply the cord. The anterior spinal artery arises from the vertebral arteries and extends from the level of the lower brainstem to the tip of the conus medullaris. It supplies the ventral surface of the medulla and the anterior two-thirds of the spinal cord. The posterior spinal arteries supply the dorsal third of the spinal cord and also originate from the vertebral arteries.
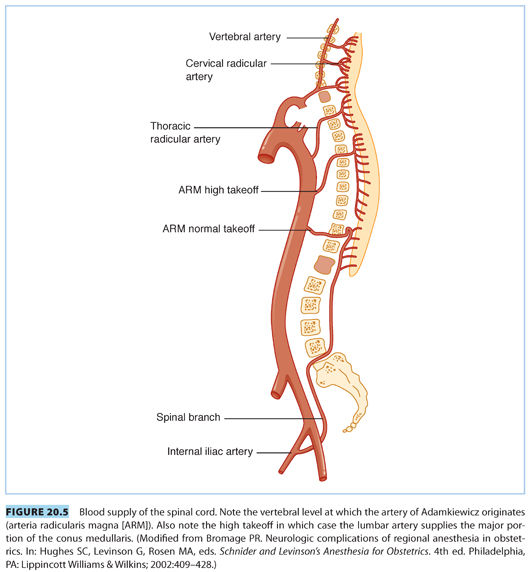
B. The artery of Adamkiewicz. At certain sites along the spinal cord, there are a number of sources of additional blood flow from other arteries: the thyrocervical trunk, intercostal arteries, and the artery of Adamkiewicz (arteria radicularis magna [ARM]).8,38 The ARM, which supplies the lower spinal cord, originates usually from the left side from one or two of the thoracolumbar segmental arteries. In most people, the origination is found between segments T9–L2.36 The ARM travels to the ventral surface of the spinal cord and fuses with the anterior spinal artery and makes a hairpin turn to course downward. This artery is often implicated in ischemic injuries to the lower portion of the spinal cord. The conus medullaris can also receive blood supply from one of the lumbar arteries originating from the internal iliac artery at the level of L5 or S1.8 However, the bulk of the conus medullaris blood supply comes from the ARM.
C. The lumbar arteries. In approximately 15% of cases, the ARM originates from spinal segments as high as the T5 level (high takeoff; see Fig. 20.5).8 In these patients, the major part of blood supply of the lower spinal cord is provided by a lumbar branch, which lies in front of the ala of the sacrum and enters the spinal cord through the L5–S1 intervertebral foramen. This branch can be compressed by the fetal head, which can lead to ischemia of the conus medullaris.
D. Diagnosis of spinal cord ischemia. Acute spinal cord ischemia is often undetectable with conventional MRI. Echoplanar diffusion-weighted MRI (DWI)8,38–40 has been used to diagnose acute spinal cord ischemia. Early DWI reveals areas of hyperintense signal indicating decreased diffusion. Follow-up MRI shows high signal on T2-weighted images and contrast enhancement at the expected levels. Echoplanar DWI38,40 may be helpful for confirmation of spinal cord ischemia in the acute stage, but follow-up images have superior spatial resolution and correlation with clinical findings. Although MRI studies within 24 hours of acute spinal cord ischemia typically are often normal, after 1 to 2 days an MRI generally shows focal cord enlargement and hyperintensity, whereas spinal cord enhancement takes 2 to 11 days.41 The MRI is also valuable in diagnosing space-occupying lesions, such as epidural hematoma or epidural abscess, or intervertebral disk herniation. CT scan is also of value in detecting space-occupying lesions and disk herniation.
CLINICAL PEARLMost serious neuropathies that occur during labor and delivery are due to the birthing process and are not the result of neuraxial blockade.
VI.Types of lesions
A. Chemical injury
Chemical injury usually results from accidental injection of an irritant into the epidural or subarachnoid space. Preservatives, such as sodium bisulfite, have been implicated in producing adhesive arachnoiditis and cauda equina syndrome. Neurologic injury may also occur as a result of local anesthetic neurotoxicity.24,30 The subarachnoid space may become obliterated from such injuries. Cauda equina syndrome has been reported to occur following the use of spinal microcatheters (28 to 32 G) for continuous spinal anesthesia. In this setting, toxicity is believed to be the result of poor mixing of concentrated local anesthetic (i.e., lidocaine 5%) with the cerebrospinal fluid (CSF), with subsequent toxic drug concentrations at the nerve roots. The U.S. Food and Drug Administration (FDA) has removed spinal microcatheters from the US market.42 Cauda equina syndrome may also occur as a result of acute intervertebral disk herniation requiring immediate surgical intervention.43
B. Direct nerve trauma
1. Paresthesia. Nerve trauma during neuraxial anesthesia is an uncommon cause of neurologic deficits. If a paresthesia with involuntary leg movement occurs, the needle or the catheter should be withdrawn immediately. Transient paresthesias perceived with dural puncture, spinal needle insertion, or epidural catheter threading are common, occuring in 5% to 20% of neuraxial blocks, with one study reporting an incidence of 14% with pencil-point needles.44 Epidural catheters with a soft polyurethane tip are associated with fewer paresthesias than the stiffer nylon catheters.45 Spinal anesthesia is associated with neurologic injury more frequently than epidural anesthesia.13,23 The anesthesiologist should document the severity and location of paresthesias. It may take 48 hours to 3 months for complete recovery from neuropathy resulting from a direct nerve trauma.7
2. Direct trauma with spinal and epidural blocks to nervous tissue may occur at the level of the spinal cord, nerve root, or peripheral nerve. Epidural placement is most likely to traumatize a nerve root. A spinal needle may contact a nerve root inside or outside the subarachnoid space, or directly injure the spinal cord. The spinal cord usually ends at the level of first lumbar intervertebral disk, but occasionally, it may extend to the L2–L3 intervertebral disk. Although the superior iliac crest denotes the L4 spinous process or L4–L5 interspace in 79% of patients, this landmark has been reported to be as high as the L3–L4 interspace in 4% of subjects.29 In pregnancy, Tuffier’s line may often be perceived as more cephalad than in nonpregnant patients. In one study, experienced anesthesiologists correctly identified the precise interspace only 29% of the time by palpation, as validated by MRI.5 In a series of >103,000 neuraxial anesthetics performed in France, two-thirds of neurologic sequelae were associated with paresthesia or pain during drug injection, suggesting direct nerve trauma; 29 of 34 neurologic complications had transient sequelae, with recovery occurring between 48 hours and 3 months.17 Intraneural injection of local anesthetics was more likely to result in prolonged neurologic deficits.
3. Spinal cord injury with spinal needles. The study from France also reported that spinal anesthesia was significantly more likely to result in both neurologic injury (5.9/10,000 vs. 2/10,000) and radiculopathy (4.7/10,000 vs. 1.7/10,000) compared to epidural anesthesia. All radicular deficits related to paresthesia from neuraxial anesthesia resolved except one.17 Combined spinal-epidural (CSE) anesthesia can be associated with minor paresthesias, but may occasionally result in severe paresthesias. Pencil-point spinal needles seem less likely to traumatize a nerve root. Reynolds46 summarized the findings from seven patients who sustained an injury to the conus medullaris during spinal anesthesia or CSE, with persistent neurologic sequelae. There was MRI evidence of spinal cord injury, and neurologic symptoms included pain, sensory deficit, foot drop, and bladder symptoms. Reynolds46 concluded that the operator inserted the spinal needle at an intervertebral space higher than L2.
4. Different degrees of nerve injury. With mild nerve injuries, only a block in conduction occurs through the damaged segment of the nerve (neuropraxia). Severe injuries lead to axonal degeneration, and axonal regeneration may never be complete, leading to total or partial loss of function in the affected area. Axonotmesis is said to occur when the axons are damaged, and neurotmesis signifies disruption of epineurium as well. Surgical repair is necessary to correct neurotmesis, and recovery may not be complete.47 The patient must be informed of the prognosis for recovery and that resolution may take several weeks, depending on the severity of initial symptoms.
CLINICAL PEARLSpinal anesthesia has a higher incidence of neurologic injury than epidural anesthesia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







