Chapter 56 Neuroimaging
Imaging Modality Overview
Ultrasound
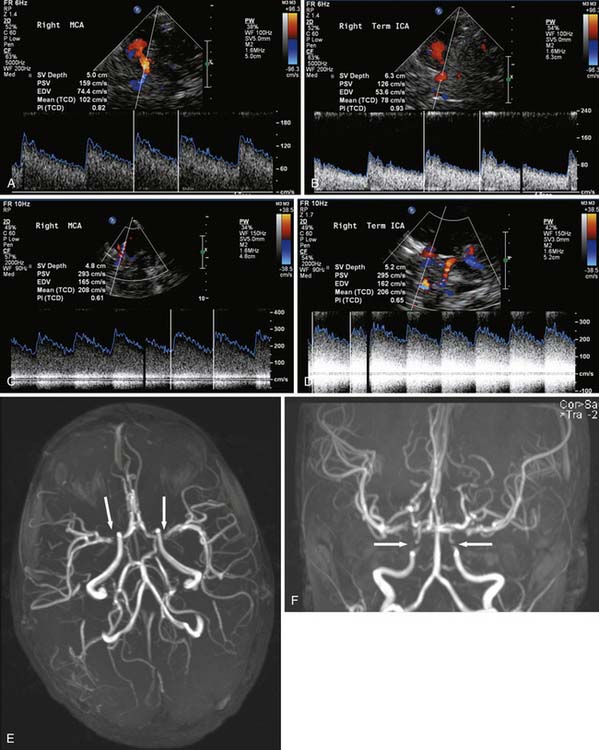
Color Doppler with the standard ultrasound machine uses the shift in frequency associated with reflection of the sound beam off a moving interface (the “Doppler shift” phenomenon) to detect motion in the image field, most commonly from blood in vessels. Those pixels with movement are assigned a color to distinguish them from pixels without movement. The color assigned (most often red and blue) is different depending on whether movement is away from or toward the transducer, and the color assigned is arbitrary so that arteries and veins are not necessarily red and blue, respectively. Color Doppler allows for some investigation of the cerebral circulation, primarily through the open fontanelle. Transcranial Doppler (TCD) uses the same Doppler shift to produce waveforms that give information about flow velocity and direction. TCD has the advantage that it can be performed through the thinner portions of the skull, primarily the temporal squamosa, in older children and adults (Figure 56-1). TCD has been used in evaluation of cerebral perfusion in patients with sickle cell disease1 and vasospasm secondary to subarachnoid hemorrhage.2 TCD is generally sensitive to stenoses greater than 50% in the central cerebral circulation, with the highest sensitivity and specificity being in the middle cerebral artery (MCA).3,4
Computerized Tomography
Computerized tomographic (CT) scanning has higher sensitivity compared with ultrasound for many intracranial pathologies, including most neurosurgical emergencies, and is not limited by closing and closed fontanelles. Imaging on modern scanners with multiarray detectors can be performed very rapidly. CT, however, requires transporting the patient from the ICU (although some portable units are available) and uses ionizing radiation (x-rays) to produce digital computer–reconstructed images based on differences in tissue density (which affects x-ray attenuation). The choice of radiation parameters, slice thickness, post-processing, and image viewing (window width and level) must be tailored to the particular clinical question to optimize the images. Bone and other calcifications have the highest density, and in decreasing density are (nonadipose) soft tissue (such as the brain), water (e.g., cerebrospinal fluid [CSF]), fat, and then air. The difference in density between bone and brain is great, whereas the difference between brain and fat is less. The density difference between gray and white matter is much smaller but is sufficient to be appreciated with the appropriate window and level. Generally, as edema develops in non-fatty tissue, there is a decrease in density. Acute blood (approximately between 12 and 72 hours old) is of higher density than brain, and CT is very sensitive in detection of acute (generally less than 1 week old) parenchymal and subarachnoid hemorrhage (SAH). CT is also useful in the evaluation of ventricular size and extra-axial collections.
Magnetic Resonance Imaging
To ensure safety, MRI-compatible monitoring and life support systems must be used. Patients need to be screened for any internal hardware or implants that would preclude scanning, such as MRI-incompatible aneurysm clips. Electronic devices such as pacemakers are rendered dysfunctional by the changing magnetic field occurring during MRI and hence patients with such devices generally should not undergo MRI (further details are available at http://www.mrisafety.com/). Injuries and deaths have been reported because of the failure to recognize these dangers. Most MRI sequences require imaging times in the range of minutes and are very susceptible to motion degradation. For patients who are unable to cooperate, deep sedation or anesthesia is often required. It also is important to screen the renal function of patients who might get an MRI because gadolinium-based contrast may need to be used. Gadolinium recently has been implicated in the development of nephrogenic systemic fibrosis in some patients with compromised renal function, which is not uncommon in the ICU setting.5
Fluid-attenuated inversion recovery (FLAIR) is a variation of T2-weighted imaging where the CSF is specifically rendered dark or suppressed so as to enhance visibility of hyperintense pathology in parenchyma, especially adjacent to normal CSF spaces. Fat is hyperintense on T1-weighted sequences and is also fairly bright on fast T2-weighted sequences, which is possible with most modern-day scanners. In some situations it is advantageous to suppress the brightness of fat, which can be specifically done at the cost of increased scan time. Gradient-recalled echo (GRE) and susceptibility-weighted MRI sequences are often utilized for detection of hemorrhage, although these sequences generally also result in more artifacts. The appearance of acute blood on CT is fairly straightforward, appearing hyperdense initially (because of the extraction of serum), becoming isodense by about a week, and appearing hypodense thereafter. The appearance of blood on MRI is much more complex, and although generally it is detectable much longer, it can be difficult to appreciate hyperacute blood (approximately less than 12 hours old) with MRI, especially subarachnoid hemorrhage. Overall, the detection of calcium and hyperacute blood can be quite difficult by MRI, and CT is often better in this regard. IV contrast is also used in MRI, with similar application with CT. In this case the contrast is a gadolinium chelate that shortens the T1 relaxation time of nearby water. The result is that the water near gadolinium is hyperintense on T1-weighted images. This effect can be used to detect BBB disruption, which is seen in various pathologies including ischemia, inflammation/infection, and status epilepticus. Malignant and some benign brain tumors also demonstrate enhanced capillary permeability with enhancement following contrast. MRI with gadolinium is about an order of magnitude more sensitive than CT using iodinated contrast in detecting BBB disruption. Some areas of the brain lack the BBB, including the choroid plexus, pituitary gland, and the median eminence, and thus they enhance normally.
Diffusion-weighted imaging (DWI) has assumed an invaluable role, particularly in the intensive care setting. This sequence is now integrated into most brain MRI examinations and is particularly sensitive to areas where brain parenchyma has sustained acute cytotoxic injury, most commonly but not limited to ischemia. DWI is sensitive to the motion of water at the molecular level.6 Detecting this molecular level motion requires strong fast gradients that have become standard on modern-day MRI scanners to allow sufficiently rapid scanning to effectively “freeze” the gross tissue motion that normally swamps this molecular level motion. Different cerebral pathologies produce different types of edema (e.g., cytotoxic, vasogenic, and interstitial). Evaluation of the molecular (Brownian) motion of water turns out to be a sensitive measure of some pathologic states, the most significant application currently being cytotoxic edema associated with the detection of ischemia.7 Cytotoxic edema causes “restriction” of water diffusion with a decrease in the apparent diffusion coefficient (ADC). This is seen as hypointensity on the calculated ADC map image but is more easily appreciated as hyperintensity on the DWI “trace” image. In contrast to cytotoxic edema, vasogenic and interstitial edema result in “increased” water diffusion and therefore an increase in the ADC. Care must be taken in interpreting the DWI trace images because they have some T2 weighting, and “T2 shine-through” can result in a focus of increased signal on DWI trace images that does not represent true restricted diffusion. Review of the calculated ADC map images should show a corresponding darkening in a focus of cytotoxic edema to confirm restricted diffusion. Also, the hyperintensity on DWI trace images may “pseudo-normalize” for a period of time several days following the ictus because of the development of associated vasogenic edema. Restricted diffusion due to infarct will generally resolve over the course of 1 to 3 weeks and allows a new infarct to be distinguished from older lesions. It also should be noted that restricted diffusion is not limited to ischemia and can be seen in other acute cerebral pathologies.
Advanced Magnetic Resonance Imaging Techniques
Perfusion MR is used in the evaluation of cerebral perfusion. The most widely available techniques use rapid scanning associated with a bolus of IV gadolinium contrast to measure first-pass changes in signal intensity. These techniques give a relative measure of perfusion, but not quantitative flow. This relative measure can be used to detect diminished cerebral blood flow in one area compared with another. Newer noncontrast MRI techniques such as arterial spin labeling generally have lower signal to noise ratio (sensitivity) but are improving and potentially quantifiable. There is some early experience with arterial spin labeling in pediatric stroke resulting from sickle cell disease.8,9
Conventional MRI is based on the signal intensities derived from the hydrogen bonded to oxygen in water and, to a lesser extent, the hydrogen bonded to carbon in fat. With the appropriate software, MR can be used as a “probe” for hydrogen bonded to other molecules (termed MR spectroscopy). The sensitivity is sufficient to detect metabolite molecules in the millimolar range, although at a much lower resolution than that used to detect water for imaging. The non-water molecules are most commonly reported as ratios of signal peaks that correspond to one of several molecules. In the brain the most common metabolite peaks detected are N-acetyl aspartate, creatine, choline, and in some physiologic states, lactate. The MRS signal is either obtained from a single voxel (usually several mL in volume; voxel is the “volume element” of the image = pixel × depth) or multiple voxels (as small as 1 to 2 mL in volume). Multivoxel MRS can be used to make low-resolution images using a technique termed chemical shift imaging for some of the more prevalent metabolite peaks. The utility of MRS is still in evolution, and newer variants of MRS are constantly being evaluated. Detection of lactate to evaluate newborn ischemia and some metabolic diseases is one of the ongoing applications and areas of research. MRS also has a role in children with mitochondrial disorders.10
Magnetic Resonance Angiography
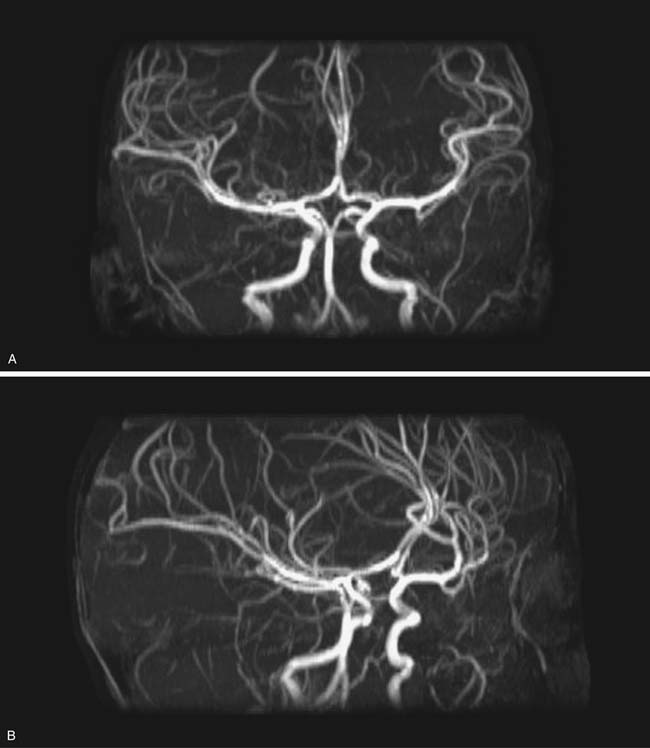
Fluid motion within tissues can be used by MRI to image flow in vessels and CSF. This imaging can be accomplished with and without contrast, although contrast techniques are generally more sensitive for vascular flow, with some newer contrast angiography sequences also providing temporal/flow information. The MR angiogram can be tailored for artery (MRA) or vein (MRV) visualization, primarily based on flow direction, and is usually most effective if the area of interest can be narrowed, for example, the circle of Willis, although newer techniques have greatly increased the area that can be covered in one scan. The maximum resolution of MRA of slightly under 1 mm is generally less than CTA, where maximum resolution can be under 0.25 mm.11 In addition to the source images (the thin angiographic sections obtained during the scan), various reconstructed images can be obtained, including 3D rotational reformations derived from maximum intensity projection reconstructions (Figures 56-2 and 56-3) and surface renderings, which also can be viewed from multiple projections. Many clinical cerebral arterial questions are now answered with MRA, and MRV of the superficial and deep venous systems has largely replaced diagnostic catheter venograms.
Catheter Angiograms
Although significant inroads have been made in cerebral vascular evaluation with both CTA and MRA, when indicated, catheter angiography remains the gold standard for most vessel imaging. Catheter angiography also provides temporal/flow data, such as appreciation of early venous drainage with arteriovenous malformations. Angiography is basically a rapid series of radiographs obtained during injection of iodinated contrast directly into the arteries or veins being imaged. Most angiography units produce digital images using digital subtraction (hence the term “digital subtraction angiography”), that is, images where the background “mask” has been subtracted, leaving an image primarily of the contrast-filled vessels. High-end modern-day units utilize biplane technology that permits acquisition of digital angiographic images from multiple projections using a single contrast injection. This technology also can be used to derive 3D rotational angiograms, images from which can be further post-processed to obtain volume rendered and surface-shaded images. Catheter angiography requires transport to and patient support in the angiography suite. Vascular access for arterial studies is usually through the femoral artery and generally has a small but “non-zero” risk of vessel injury, including dissection and embolization. In very young patients, injury to the femoral artery is of greater concern, and although any acute risk to the limb is extremely uncommon, relative diminished leg growth in some cases has been documented. Risks of neurologic complication following cerebral angiography are small but do exist, with the reported incidence of permanent defects typically ranging from about 0.5% to 0.07%.12,13 Therapeutic endovascular procedures carry higher risks but generally are in lieu of riskier neurosurgical procedures or at times are the only avenue of treatment.
Nuclear Medicine
Most nuclear medicine studies involve injection of a very small amount of radioactively labeled substance (radiopharmaceutical tracer compound or radiotracer) and either follow the physiologic uptake and metabolism of the radiotracer or the movement of the radiotracer with a gamma camera.
Preterm and Term Neonate Imaging
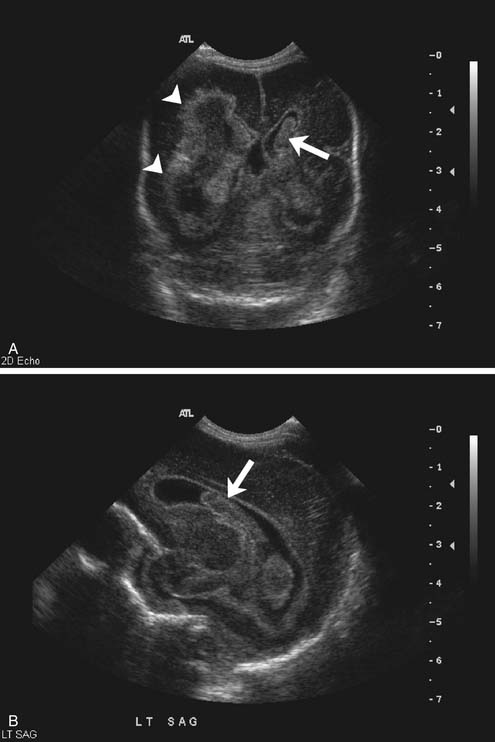
In the premature infant, ultrasound remains the primary modality for detection and follow-up of germinal matrix hemorrhage and to detect intraventricular extension, and in the neonate, it remains the primary modality to evaluate for hydrocephalus (Figure 56-4). Furthermore, assessment can be made for white matter injury including periventricular leukomalacia, especially the cystic form, although MRI will be more sensitive for noncystic periventricular leukomalacia, and MRI in preemies performed at about the third week of life has been reported as predictive of outcome at term.14 Routine screening cranial ultrasound has been recommended for all infants younger than 30 weeks’ gestation between day 7 and 14, optimally repeated at 36 to 40 weeks’ gestation.15 Moderate to large parenchymal and extraaxial areas of bleeding also can be detected with ultrasound. As previously mentioned, smaller extraaxial collections, especially laterally along the cerebral convexities, can be missed with ultrasound, as can small parenchymal hemorrhages.
As noted in Chapters 57 and 62, understanding of hypoxic-ischemic encephalopathy (HIE) in children is complicated by the developmental status of the child. The pattern of injury seen is determined by the characteristics of the insult and the maturational state of the brain. Metabolic demands and regions of selective vulnerability evolve during development. Grayscale ultrasound, which is widely used for intracranial imaging in the neonate, is relatively insensitive to acute changes associated with HIE.16 As edema develops, usually after several hours, increased echogenicity of brain parenchyma can be seen, but this measure is nonspecific and relative and often is difficult to appreciate. Blood associated with a hemorrhagic infarct also will appear as increased echogenicity. As mass effect develops secondary to edema, ventricular and sulcal effacement can be seen. Later, vascular and perivascular mineralization can result in linear thalamic and basal ganglia echogenicities (lenticulostriate vasculopathy or mineralizing angiopathy).17 Ultrasound Doppler evaluation of newborn ischemia has shown some utility. Brain perfusion can be investigated by determining the resistive index (RI). RI in the normal neonate (0.75 ± 0.1) is higher than that seen in the older infant before (0.65 ± 0.5) and after (0.55 ± 0.5) fontanelle closure.16 An increase in the RI with a decrease in the peak systolic velocity and end diastolic velocity in infants with HIE within the first 12 hours of life as measured in the anterior and middle cerebral arteries correlated with a poor prognosis at 1 year of life. A decrease in RI to less than 0.60 at 12 hours (anterior and middle cerebral arteries) and 24 hours (all insonated arteries, including basilar artery) after neonatal asphyxia, which is thought to be a result of the decreased vascular tone associated with loss of autoregulation, has been associated with a poorer outcome at 12 to 18 months of life.18,19 Approximately half of these patients with low RI scores have normal grayscale images. There is a host of other reasons for low RI scores, however, including cardiac disease, extracorporeal membrane oxygenation, ongoing hypoxia, hypercapnia, and technical issues. It also should be noted that increased fontanelle pressure can increase the RI measure by 20%, hence there is considerable user dependence to this application. If hyperemia persists, and as HIE evolves, cytotoxic edema increases and leads to increased intracranial pressure and increased RI measurements. A high RI score on the first day of life with evidence of neonatal insult suggests an in-utero injury. Although some centers have continued to pursue use of ultrasound, much of this evaluation has been supplanted by MRI.
CT detection of acute ischemic injury also depends on edema resulting from the injury. Edema is seen as decreased attenuation and loss of gray-white differentiation, usually several hours post ictus.20 Small or early infarcts can be missed with CT, and detection of ischemia in the newborn is made more difficult by the generally lower attenuation of the relatively “watery” unmyelinated newborn brain. For this reason, in some instances of neonatal ischemic injury, there may actually be an increase in the attenuation of this “watery” unmyelinated white matter because of an outpouring of serum proteins from damaged blood vessels. As brain swelling develops in the first few days, there can be a loss of CSF spaces seen as ventricular compression, sulcal effacement, and loss of perimesencephalic cisterns. Acute thrombotic stroke associated with arterial thrombosis can at times be appreciated acutely as a hyperdense artery, most commonly the middle cerebral artery on noncontrast CT. Acute hemorrhage, such as with hemorrhagic arterial (from reperfusion) or venous infarcts, will be hyperdense initially on CT, evolving to isodense over the first week.
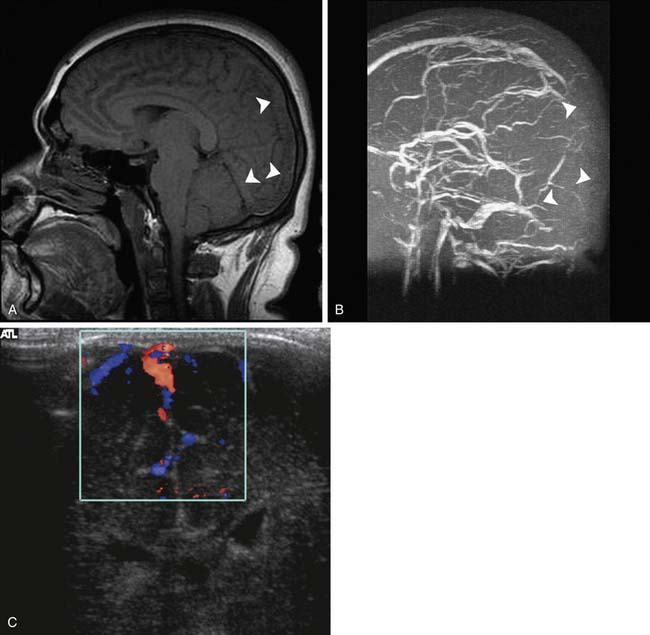
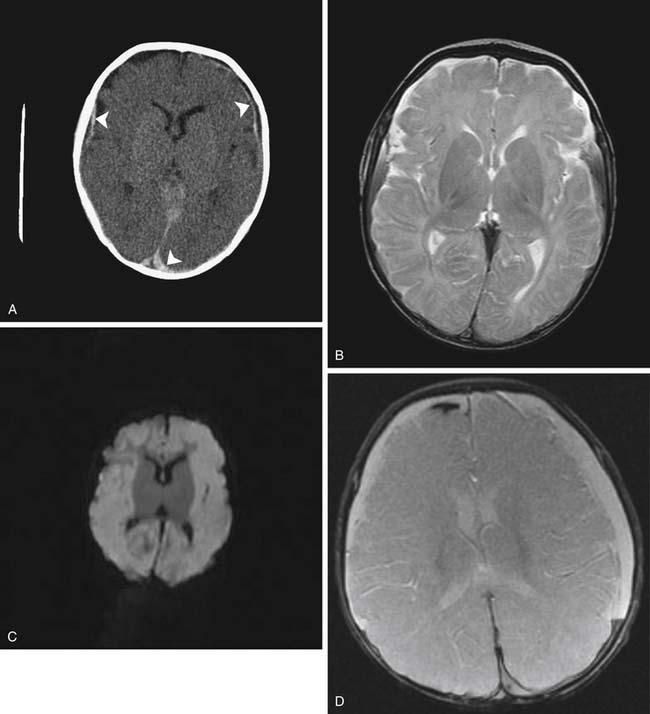
Standard MR sequences exploiting T1 and T2 relaxation times also depend on the development of edema to appreciate acute ischemic injury, which results in hyperintensity on T2-weighted and FLAIR images and hypointensity on T1-weighted images. This change typically takes at least several hours to develop, and although generally more sensitive than CT in adults, evaluation of the newborn is again made somewhat difficult by the lack of myelination. As a result ischemia sometimes can be more conspicuous on CT than on T1- or T2-weighted MRI (Figure 56-5). The FLAIR sequence has proven to be more sensitive than T1 and T2 to ischemic changes in older myelinated children and adults. DWI is more sensitive than T2 and FLAIR sequences and correlates well with at least short-term neurologic outcome in neonates and infants.21 These sequences, as previously discussed, have been shown to be acutely sensitive to the cytotoxic edema associated with ischemia.7 This cytotoxic edema results in diminished diffusion of water in the affected area. DWI in experimental models can detect ischemia in minutes after onset as a region of restricted diffusion.22,23 These sequences have now become widely implemented in adult and pediatric neuroimaging, where differentiation of acute ischemic stroke from other neurologic disorders permits appropriate implementation of stroke therapies. The restricted diffusion associated with ischemia evolves over a 1- to 3-week period, at which time the diffusion image usually normalizes. If sufficient tissue destruction has occurred, the diffusion ultimately will increase because of the greater amount of free water following necrosis. This change in diffusion is also useful in distinguishing a new stroke (which will show decreased diffusion) from an older lesion (which will have increased diffusion), whereas both lesions may be of similar signal intensity on standard T1 and T2 imaging (Figure 56-6).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree