Fig. 42.1
Local anesthetic requirements for pregnant vs. nonpregnant patients (With permission from Oxford University Press [11])
Several factors may cause pregnant patients to be more sensitive to local anesthetics. As mentioned above, reduction in epidural and CSF volume may be factors. Other factors such as hormonal milieu may act to make individual neural elements more sensitive to local anesthetics [13].
Pain Pathways
Labor pain has both visceral and somatic components that evolve as labor progresses. During the first stage of labor, the visceral component dominates. Uterine contractions result in myometrial ischemia and the release of nociceptive mediators such as bradykinin, potassium, and serotonin. In addition, mechanoreceptors are stretched as the cervix dilates. Pain impulses are transmitted via the sympathetic pathways in the paracervical region, through the pelvis to the hypogastric plexus, entering the lumbar sympathetic chain that enters the dorsal horn of the spinal cord at the level of T10 to L1. During the second stage, a somatic component caused by a stretch of the perineum is added. These enter the spinal cord at the level of S1–S4 (Fig. 42.2).
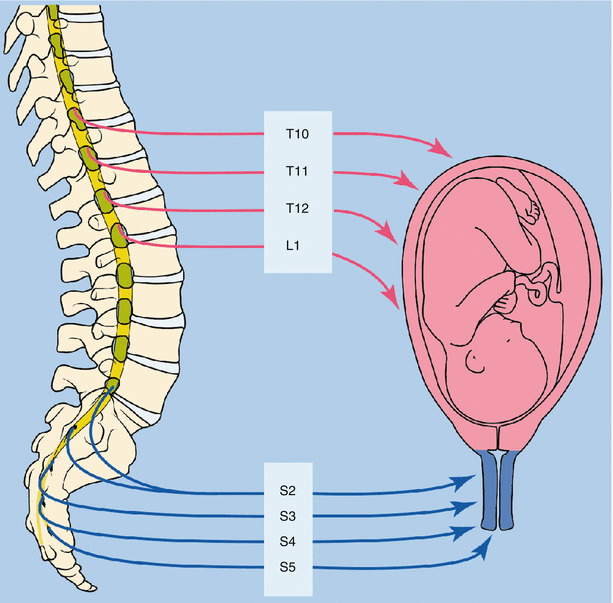

Fig. 42.2
Pain pathways for parturition (With permission from Danilo Jankovic)
Goals of Therapy
Childbirth is a multidimensional experience, and when considering treatment, one must strike a balance between pain relief and other aspects, such as physical, emotional, psychological, sociological, and other needs. For example, some women may consider analgesia unsatisfactory if it results in reduced ability to participate in the second stage of labor. A list of the characteristics of an ideal labor analgesic can be found in Table 42.1. Neuraxial analgesia can come very close to this ideal when properly conducted.
Effective Pain Relief
Neuraxial analgesia is more effective than other methods. A recent meta-analysis of 17 randomized controlled trials comprised of 6,019 patients reported that 1 % of women required additional means of analgesia when neuraxial analgesia was used compared to 22 % for other methods [14]. Further, in an earlier meta-analysis of seven randomized trials comprised of 2,000 patients reported significantly reduced visual analog pain scores in patients who received neuraxial analgesia compared to those who received parenteral opioids. In the first stage, the difference was 40 mm (on a 100-mm scale), and in the second stage, it was 30 mm. Both were highly statistically and clinically significant [15].
Maternal Safety
When properly conducted, neuraxial analgesia has an excellent maternal safety record. The incidence of severe neurological complications is lower in parturients that in nonpregnant patients receiving neuraxial anesthesia in the perioperative setting [16]. When placing a neuraxial block, the anesthesiologist must be aware of the lumbar interspace and ensure that it is below the termination of the spinal cord. Other precautions, such as meticulous sterile technique, a system to ensure the correct drug is injected, etc., should be observed in the same fashion as in the operating room. There are a number of special precautions that should be taken to minimize the possibility of maternal injury. These include:
1.
Avoid hypotension. This can occur because of pressure from the uterus on the inferior vena cava causing a reduction in preload. Hypotension may result in maternal lightheadedness or fainting and can be avoided by displacing the uterus (usually to the left side) off the vena cava. Caval compression becomes clinically significant when the fundus of the uterus can be palpated at the level of the umbilicus, usually after the 20th week of gestation. A vasopressor such as ephedrine or phenylephrine should be immediately available to treat hypotension. A large bolus of fluid prehydration is likely unnecessary before initiation of low-dose neuraxial analgesia [17, 18].
2.
Post-dural puncture headache. This can occur in up to 1.6 % of patients who receive epidural analgesia. The incidence of accidental dural puncture with a large-bore epidural needle may be reduced by ensuring a controlled entry into the epidural space and advancing the needle when the patient is not having a contraction. A recent systematic review of randomized controlled trials did not find any maneuvers that were effective in reducing accidental dural puncture [19]. When deliberately puncturing the dura with a spinal needle during a combined spinal–epidural technique (CSE), dural puncture headache can be minimized by using the smallest gauge needle possible and a non-cutting needle [20].
3.
Avoid accidental intravenous or intrathecal injection of local anesthetic. Fortunately, the doses of local anesthetic used for neuraxial analgesia for labor are very low. Some clinicians prefer to perform a “test dose” before the initial loading dose. This may include a combination of epinephrine that may be detected as maternal tachycardia when injected intravenously and a small dose of lidocaine that would immediately cause profound analgesia and motor block if injected intrathecally. Unfortunately, this combination does not achieve its goals. The baseline heart rate during labor is variable, and there may be confusion between the tachycardia caused by the test dose and the tachycardia caused by contraction pain. The lidocaine component may be effective in demonstrating an intrathecal injection, but it also causes unnecessary motor block when injected into the epidural space [21]. It may be preferable to fractionate the loading dose so that each fraction becomes a “test dose.”
Fetal and Neonatal Safety
While providing analgesia, it is important to cause as little disturbance to the fetal physiology as possible. In addition, any drug given to the mother will be transferred to the maternal circulation and ultimately to the fetal circulation. These drugs may have a direct or indirect effect on the fetus and newborn. Drugs that remain in the maternal circulation will be present in breast milk, providing additional exposure to the newborn.
Local anesthetics, in the doses given for labor analgesia, do not cause any important direct physiologic or pharmacologic effects on the fetus [22]. Indirect effects may be more important and may be beneficial or harmful. Neuraxial analgesia that results in hypotension causes abnormal fetal heart rate patterns that are reversed when hypotension is treated. Severe labor pain increases maternal ventilation and hypocarbia resulting in acute respiratory alkalosis. This may result in a shift in the oxygen dissociation curve and reduced oxygen delivery to the fetus. Severe pain may also cause an increase in circulating catecholamines that may result in discoordinated labor and reduced uteroplacental perfusion [23]. Pain relief reverses these changes. However, if pain relief is too rapid, some authors suggest that uterine tetany may result in an acute, short-lived increase in uterine tone, restricting blood flow. Thus, fetal bradycardia is observed more often when a CSE is given for labor analgesia compared to epidural alone. It should be noted that the effect is transient and does not increase the incidence of Cesarean section for fetal distress or affect neonatal outcome [23].
Opioids, such as fentanyl and sufentanil, are often given combined with local anesthetics either in the epidural or subarachnoid space. These have few direct effects on the fetus because the dose is low. If there is maternal respiratory depression, this may indirectly lead to fetal acidosis and hypoxia. Whether or not opioids, when given in the epidural space, can cause breastfeeding problems is controversial. Unfortunately, measurement of breastfeeding success is very difficult because there are no standard measures and there are multiple factors that may impact on the outcome. These include maternal health, other pharmacologic interventions, surgical interventions, sociologic considerations, and hospital policies [24]. The effect of neuraxial analgesia on breastfeeding is discussed in detail below.
Progress of Labor
Whether or not neuraxial analgesia causes maternal harm by impeding progress of labor has been controversial. Uncontrolled cohort studies consistently show a strong association between epidural analgesia and an increased incidence of Cesarean section, even when known confounding demographics are considered [25]. Women who have a prolonged and more painful and latent phase of labor are more likely to require obstetric intervention compared to those that do not [26], likely because of an increased incidence of dystocia in this group [27]. Therefore, increased pain may be a marker for poor obstetric outcome, and these patients are more likely to request epidural analgesia.
Over the last 20 years, there have been numerous randomized controlled trials comparing neuraxial to non-neuraxial analgesia for labor. Patients in the study group received either epidural or combined spinal–epidural analgesia. Patients in the control group received parenteral opioids or non-pharmacologic interventions. A recent meta-analysis comprised of 38 studies, and almost 10,000 patients concluded that neuraxial analgesia did not increase the risk of Cesarean section (relative risk = 1.10, 0.97–1.25) but may be associated with an increased incidence of instrumental vaginal delivery (relative risk = 1.42, 1.28–1.57). They also found that the duration of the second stage of labor was prolonged by about 13 min.
It is not clear whether or not mode of analgesia was the direct cause of the increased incidence of instrumented vaginal delivery. In particular, the presence of neuraxial blockade may induce behavioral changes in the obstetrical team. For example, epidural analgesia facilitates forceps delivery. At least one author described an excess of instrumented vaginal delivery in their parturients with epidurals for the purpose of resident training [28]. As discussed in detail below, the rate of instrumental vaginal delivery can be minimized by using low concentrations of local anesthetic to maintain analgesia [29].
Maternal Side Effects
The main focus of research into neuraxial analgesia for labor concentrates on reduction of maternal side effects. Effective maneuvers for prevention and treatment will be discussed below.
Pharmacology
The main drugs used in the epidural and subarachnoid space for labor include local anesthetics, opioids, and epinephrine. Clinical trials using other adjuvants such as clonidine, magnesium, and neostigmine have been reported, but at this time, none of these are used outside of the research setting and will not be considered further.
Local Anesthetics
Long-acting, amide local anesthetics are most commonly used for labor. Bupivacaine and ropivacaine are the only anesthetic agents available in North America. Levobupivacaine is available in most other parts of the world.
Lidocaine was the first local anesthetic used for labor analgesia, but its use was limited because of the high incidence of motor block and tachyphylaxis as labor progresses. Bupivacaine, first manufactured in 1957, was superior and became the most common local anesthetic used for labor epidural analgesia. In 1979, Albright reported a case series of six patients who suffered cardiac arrest because of accidental intravenous injection of a large dose of bupivacaine or etidocaine, calling the safety of these long-acting local anesthetics into question [30]. Ropivacaine and levobupivacaine were developed in an attempt to increase safety. It should be noted that cardiac toxicity has not been reported with any local anesthetics in the low doses currently recommended for initiation and maintenance of labor analgesia.
There have been numerous studies comparing ropivacaine to bupivacaine for labor in terms of efficacy, side effects, and labor outcome. It should be noted that ropivacaine is about 60–75 % as potent as bupivacaine when given as a bolus for initiation of labor [31]. When potency is considered, there is very little difference between the two drugs. In particular, both provide excellent analgesia and do not interfere with the progress of labor. In patients who received epidural analgesia for more than 6 h, there is a statistically significant increase in the incidence of motor block in the lower extremities [32]. However, the effect is small. At this time, ropivacaine tends to be somewhat more expensive than bupivacaine and economic factors, rather than clinical factors may decide which drug to use.
There is less information about levobupivacaine than either bupivacaine or ropivacaine. The potency of levobupivacaine seems to be about the same as bupivacaine. Block characteristics including the incidence of motor block and analgesic efficacy seem to be similar to bupivacaine [33].
Opioids
Lipid soluble opioids such as fentanyl and sufentanil bind to receptors in the spinal cord and work at that level, rather than through systemic mechanisms [34, 35]. These are often combined with local anesthetics for both initiation and maintenance of labor analgesia. While other opioids have been studied, none are commonly used in clinical practice and will therefore not be considered further (see reference [36] for a full review).
When fentanyl is used for initiation of analgesia with 20 ml of bupivacaine, the median local anesthetic concentration (MLAC) for 50 % effectiveness was reduced from 0.064 to 0.034 %, demonstrating significant local anesthetic-sparing effect [34]. When used for maintenance [35], a similar local anesthetic-sparing effect was demonstrated. When used for initiation of analgesia, the addition of sufentanil increases the speed of onset (10.3 vs. 8.7 min) [37]. Clinically, the lowest, clinically effective concentration of local anesthetic should be used to reduce the intensity of motor block and possibly reduce the incidence of operative vaginal delivery [29].
Of interest, epidural fentanyl alone will provide moderate analgesia early in labor, but it is inadequate for the late first stage and second stage of labor [38]. Large doses of fentanyl cause severe pruritus, nausea, and vomiting. Significant systemic uptake also causes maternal sedation and possibly neonatal depression. For these reasons, lipid soluble opioids should be used with local anesthetic to take advantage of the synergy between the two classes of drug.
In summary, numerous studies have shown the benefit of combining low concentrations of long-acting local anesthetics such as bupivacaine or ropivacaine with lipid soluble opioids. Low doses of opioids should be used to reduce the incidence and severity of common side effects such as pruritus, nausea, and sedation. Low doses of opioids do not appear to affect neonatal outcomes.
Epinephrine
Epinephrine is sometimes used as an intravenous test for accidental intravenous injection through the epidural catheter. For this purpose, 15 mcg is often combined with 45–60 mg of lidocaine. The test is based on detecting maternal tachycardia. Unfortunately, maternal heart rate is extremely variable during labor and detection of tachycardia is often “false positive.” This would lead to removal and reinsertion of the epidural catheter unnecessarily. While this test has been recommended by some investigators, most obstetric anesthesiologists have abandoned this practice [39].
Some clinicians add a small amount of epinephrine to local anesthetics to prolong duration of action and to reduce local anesthetic absorption. These advantages are not relevant for labor analgesia. In most cases, initiation of labor analgesia is immediately followed by an infusion eliminating the need for prolonged duration analgesia. Typically, a very low cumulative dose of local anesthetic is used, even in prolonged labor so that local anesthetic absorption is not an issue. Epinephrine may have some harmful effects such as transiently increasing maternal heart rate and reducing uterine activity. Finally, there may be an increased incidence of lower limb motor block [40].
Initiation of Analgesia
Initiation of analgesia can be accomplished either by using a standard epidural technique or a combined spinal–epidural (CSE) technique (see below).
Epidural analgesia is usually initiated with a low concentration of local anesthetic with or without fentanyl or sufentanil. Typically, 15–20 ml of 0.080–0.125 % bupivacaine (or the equivalent concentration of ropivacaine) can be given with or without up to 25 mcg of fentanyl in divided doses. Low concentrations of local anesthetic result in a reduced incidence of operative vaginal delivery compared to 0.25 % bupivacaine [41].
Initiation of labor analgesia using a CSE technique has become a popular method. This is usually done using a needle-through-needle technique. The epidural space is found with an epidural needle using a loss-of-resistance technique. Then, a long spinal needle is advanced through the epidural needle into the cerebrospinal fluid (CSF). The spinal needle is removed and an epidural catheter is placed. No additional medication is required at this time, but a syringe should be attached to the epidural catheter to aspirate for CSF. This is done in order to ensure the epidural catheter has not accidentally been passed into the intrathecal space. The epidural catheter is secured and maintenance of analgesia can begin.
Proponents of the technique note the very rapid onset of analgesia without the attendant motor block. This technique takes advantage of the synergistic analgesia obtained using a low dose of intrathecal local anesthetic with a lipophilic opioid such as fentanyl [42]. Some clinicians are concerned that rapid pain relief may lead to an increase in uterine tone and result in an excess incidence of fetal bradycardia.
A recent meta-analysis comprised of 27 randomized controlled trials and 3,274 women compared the effectiveness and side effects of epidural analgesia and CSE for labor [43]. CSEs had a faster onset of analgesia (5.4 min, 95 % confidence interval 3.6–7.2). One small study noted that all patients (n = 50) reported good analgesia at 10 min after a CSE while only 50 % had good analgesia after a low dose epidural. However, the incidence of successful analgesia was similar in both groups at later time points [44]. Low-dose epidural analgesia did not impair mobility, and there was no difference between groups. The incidence of pruritus was significantly higher in the CSE group. Whether labor analgesia is initiated with a CSE or epidural, maternal satisfaction rates are high and there is no difference between groups. There were no differences between groups for any other maternal side effects such as dural puncture headache, nausea, vomiting, or hypotension. Importantly, there is no difference in the incidence of spontaneous vaginal delivery or Cesarean section between the two groups, nor was there any difference in any measured neonatal outcomes. There was a statistically significant increase in the rate of operative vaginal deliveries, but the difference between groups was small, except when high concentrations of local anesthetic were used to initiate analgesia [41]. There was no difference between groups when only low-dose epidurals were compared to CSEs. The authors of the meta-analysis noted that there were insufficient data to comment on the maternal safety of CSEs since the incidence of such complications a meningitis and nerve damage are very low [43].
Fetal bradycardia has been observed in relation to CSEs. An early systematic review [45] and a large randomized controlled trial [23] concluded that the incidence of fetal bradycardia may be increased after CSE compared to epidural. A recent randomized controlled trial compared the incidence of abnormal fetal heart rate patterns in nulliparous patients who received epidural analgesia or CSE. The investigators noted that there was an increased incidence of abnormal fetal heart rate patterns in both groups after the initiation of analgesia. There was no difference between groups in the rate of Cesarean section or neonatal outcomes [46].
Maintenance of Analgesia
The use of a continuous epidural catheter allows provision of analgesia for the duration of labor. Low concentrations of long-acting local anesthetics such as bupivacaine or ropivacaine combined with lipophilic opioid provide analgesia throughout labor. Intermittent boluses of local anesthetic by clinicians can be an effective strategy for maintenance of analgesia, but it is time-consuming. A continuous infusion of local anesthetic provides a more constant level of analgesia throughout labor. More recently, patient-controlled epidural analgesia (PCEA), in addition to a low-volume continuous infusion, has become the preferred technique. A meta-analysis of nine studies comprised of 640 patients compared to continuous epidural infusion alone to PCEA. Patients in the PCEA group had a reduction in the number of interventions required by clinicians, a reduction in the amount of drug given, and a reduction in the incidence of motor block of the lower extremities. There was no difference between groups in other analgesic outcomes, maternal satisfaction, progress of labor, or neonatal outcome [47].
When using PCEA, the clinician must determine the concentration of local anesthetic, the size of the allowed bolus dose, the lockout interval between doses, and the presence of a background continuous infusion. While there is no single “best” setting, there are some principles that can guide dosing.
Dilute solutions of local anesthetic should be used. Concentrations of bupivacaine between 0.0625 and 0.125 % are commonly used. Low concentrations require the addition of fentanyl or sufentanil to be effective. Higher concentrations of local anesthetic do not increase the analgesic efficacy but do lead to an increase in motor block of the lower extremities [48]. In addition, higher concentrations make operative vaginal delivery and prolong second stage of labor more likely [29].
There is a wide range of patient-initiated bolus doses that is safe and effective. It is important that the bolus should not be too large in case of the unlikely event that the epidural catheter migrates intravenously or intrathecally. The usual dose range, using the concentrations outlined above, is between 4 and 10 ml, although up to 20 ml has been reported [48].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








