Fig. 37.1
Anatomy of skin and soft tissue and infectious processes associated with each layer
Epidemiology
Incidence
The incidence of NSTIs in the United States has been estimated from large administrative databases and has been noted to have increased since the 1980s [3, 4]. Whether the increase represents a true rise in the number of infections or simply better identification and reporting of NSTIs is unclear. An analysis of medical claims data from 1997 to 2002 reported the incidence rate of NSTIs, or the probability of developing the disease over a specific period of time, as 0.04 per 1,000 person-years [5]. A review of more than 28 million patients in the Nationwide Inpatient Sample (NIS) database in the years 2001 and 2004 identified a total of 10,940 or 0.04% of hospitalized patients as having an NSTI [6]. Although rare, it is estimated that clinicians, whether surgeons or primary care physicians or specialists, will encounter at least one NSTI patient in their lifetime [7].
Classification
There are several methods for describing NSTIs, although there is no standard classification system. NSTIs can be described by their depth of invasion (Fig. 37.1); necrotizing fasciitis is characterized by pathological findings at the level of the subcutaneous fat (i.e., thrombosed vessels) and deep fascia (i.e., necrosis). NSTIs can also be classified by their anatomic location (i.e., Fournier’s gangrene for NSTIs of the perineum). Another method for describing NSTIs is based on their microbiology: Type I, II, or III. Type I NSTIs are the most common type, accounting for 55–75% of infections. They are polymicrobial and include organisms such as gram-positive cocci (i.e., Staphylococcus aureus), gram-negative bacilli (i.e., Escherichia coli), and anaerobes (i.e., Clostridium and Bacteroides species). They have been associated with multiple predisposing factors including surgical procedures, diabetes, and peripheral vascular disease. Type II NSTIs are caused by Group A beta hemolytic Streptococci with or without S. aureus. These infections are less common than Type I infections and can occur in young, healthy individuals. Type III NSTIs have been attributed to Vibrio species by some authors and to Clostridium species by other authors [8].
An alternative classification system was proposed by Bakleh et al. based on histopathologic findings [9]. They proposed three stages based on combinations of inflammatory response and gram-stain results. Grades of the inflammatory response were characterized by the degree of neutrophilic infiltration and presence of necrosis or microabscesses. The histopathologic stages correlated with mortality, although only unadjusted analyses were performed due to small sample size.
Risk Factors
Although there are multiple risk factors for NSTIs that include medical comorbidities and other factors, NSTIs often develop in young, healthy hosts. Comorbidities include diabetes mellitus, peripheral vascular disease, obesity, chronic renal failure, cirrhosis, heart disease, acquired immunodeficiency syndrome (AIDS), and immunosuppression. Injection drug use and alcoholism are associated with NSTIs as well. Infections may develop as a result of insect bites, abscesses, recent trauma, or surgery [3, 10].
Microbiology
As previously described, NSTIs may be polymicrobial or monomicrobial depending upon the patient’s comorbidities, risk factors, and clinical setting. One study of patients from the late 1980s and early 1990s found an average of 4.4 organisms per infection [11]. Cultures may identify gram-positive and gram-negative bacteria, aerobic and anaerobic bacteria, and fungi. Historically, monomicrobial NSTIs were attributed to Group A Streptococcus (GAS), Clostridium species, and Vibrio species, but as described as follows, any number of microorganisms may cause monomicrobial NSTIs.
The two most common gram-positive cocci isolated from patients with NSTIs are Staphylococci and Streptococci [1, 11]. S. aureus is the most common pathogen present in serious soft tissue infections in North America, Latin America, and Europe [12]. Over time, its virulence and resistance has changed; there has been a concomitant decrease in infections caused by methicillin-sensitive Staphylococcus aureus (MSSA) and an increase in infections caused by methicillin-resistant Staphylococcus aureus (MRSA) [13]. Furthermore, there has been an increase in the prevalence of community acquired MRSA (CA-MRSA), which was first described in the 1990s [14]. Initially CA-MRSA infections were primarily present only in specific sub-populations such as prisoners or sports participants, but now CA-MRSA is on its way to becoming the predominant strain of MRSA in hospitals [15]. Similarly, CA-MRSA was not previously common in patients with NSTIs [16]. In 2005, Miller et al. described 14 patients with NSTIs and positive cultures for CA-MRSA, 12 of who had monomicrobial infections [16]. These patients had risk factors that included medical comorbidities such as diabetes and hepatitis, history of injection drug use, homelessness, and prior MRSA infection. All of the infections were due to the USA300 clone and had similar genotypes including the presence of the Panton–Valentine leukocidin (pvl) gene, which encodes an exotoxin that causes leukocyte destruction. Several series of patients have reported high rates of MRSA NSTIs, although genotyping was not performed in all of the series [17–20]. In one case series, MRSA was the most frequent cause of monomicrobial NSTIs [18]. There is a suggestion that mortality may not be as high in patients with CA-MRSA, but because of its increasing prevalence, empiric coverage should be started in patients with suspected NSTIs [16–20].
Streptococcus pyogenes is a type of Group A beta-hemolytic Streptococcus (GAS) that can cause a spectrum of diseases from bacterial pharyngitis to necrotizing fasciitis and myositis to toxic shock syndrome. In a European population-based study, the crude rate of S. pyogenes infection was 2.79 per 100,000 population [21]. Eight percent (308 patients) of all of the cases were diagnosed with necrotizing fasciitis, of which 50% were associated with toxic shock syndrome (TSS). Streptococcal TSS has been reported to be an independent predictor of mortality [22]. Risk factors for GAS infections include comorbidities such as liver disease or underlying malignancy and behaviors such as injection drug use, but these infections can also occur in healthy immunocompetent patients as well [23]. GAS NSTIs have a predisposition for the lower extremities and tend to spread rapidly. These organisms have a number of factors that contribute to their virulence including M protein, which facilitates attachment to the host cells and prevents bacterial phagocytosis, enzymes that facilitate the spread of infection and that prevent the migration of neutrophils to the site of infection, and superantigens that stimulate a pro-inflammatory response [8].
Gram-negative rods have been associated with NSTIs including Klebsiella species, Enterobacter species, Pseudomonas and Aeromonas, Vibrio species, Acinetobacter species, Eikenella corrodens, and Citrobacter frundii [1, 11]. Liver disease is a risk factor for NSTIs due to gram-negative rods, particularly Vibrio, Klebsiella, and Aeromonas [24]. Furthermore, these gram-negative rod NSTIs appear to have a higher prevalence in Asian countries [24]. Vibrio infections occur in immunocompromised hosts such as those with cirrhosis, diabetes mellitus, adrenal insufficiency, and chronic renal insufficiency; they are associated with contact with seawater or ingestion of raw seafood [24–28]. These infections may have an atypical presentation; increased level of suspicion should occur in these patients, particularly when hemorrhagic bullae are present given an increased associated mortality [25, 26]. Klebsiella NSTIs are more common in Asia, but has been reported to have been acquired nosocomially in a patient with underlying malignancy in the Western hemisphere [29].
Clostridum is a genus of gram-positive bacteria that are obligate anaerobes. Multiple species including Clostridium perfringens have been identified in NSTIs [1]. Clostridial infections may cluster in areas with heavy injection drug use. For example, King County, Washington, has a high prevalence of drug users who inject heroin. In a review of 10 years’ of autopsies of patients who died due to NSTIs, clostridial infections were identified as being significantly associated with injection drug use of black tar heroin [30, 31]. Different species were noted including Clostridium sordellii. A retrospective review of patients treated in Seattle, Washington, similarly identified an association between clostridial infections and injection drug use. Furthermore, clostridial infections were significantly associated with an increase in mortality and limb loss [30]. NSTIs caused by Clostridium septicum are often associated with an underlying colonic malignancy [32, 33].
Fungi (i.e., Candida species) may also be found in both polymicrobial and monomicrobial NSTIs. There have been case reports of monomicrobial NSTIs due to Aspergillus [34, 35]. Zygomycotic NSTIs from Apophysomyces have been reported in trauma patients and in immunocompetent hosts [36–38]. Cryptococcocal NSTIs have also been reported, largely in immunocompromised patients [39, 40].
Pathophysiology
Spread of pathogens that cause NSTIs occurs through the production of a variety of endotoxins and exotoxins, many of which have already been mentioned. Toxins may cause tissue destruction, ischemia, and necrosis; endothelial damage, which results in increased tissue edema and impaired capillary blood flow; increased escape from host defenses such as phagocytosis and neutrophil infiltration at the site of infection; and activation of the coagulation cascade, which may cause vascular thrombosis and worsened tissue ischemia [3].
Clinical Presentation
NSTIs can be difficult to distinguish from other non-necrotizing infections. Early manifestations may include swelling, erythema, and warmth, which are nonspecific findings that are also present in patients with cellulitis (Fig. 37.2). Pain out of proportion to physical exam may be present. By the time NSTIs become clinically apparent and patients manifest “hard signs,” the associated morbidity and mortality are increased because of the delay in diagnosis [41–43]. Hard signs include late skin manifestations such as bullae, crepitus, or skin necrosis (Figs. 37.3 and 37.4). Wang et al. performed an observational study of patients and developed a staging system based on the time course of symptoms and signs (Table 37.1) [44]; such hard signs are classified as Stage III or late findings. Furthermore, NSTI patients may present with hemodynamic instability and organ failure; the number of dysfunctional organ systems at admission is predictive of mortality [45].
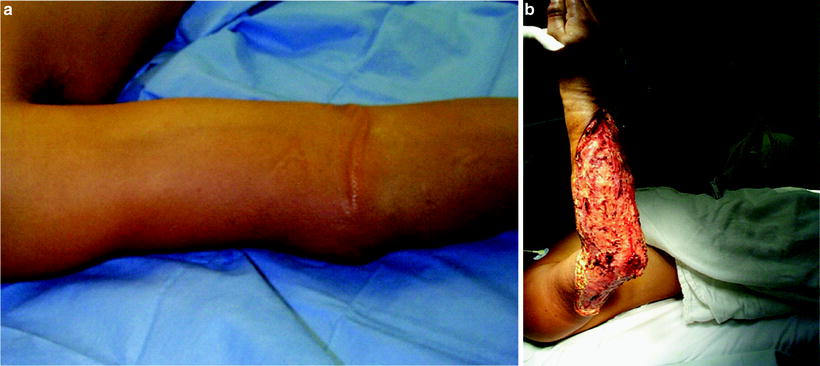
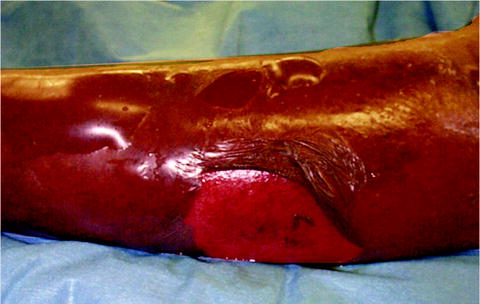
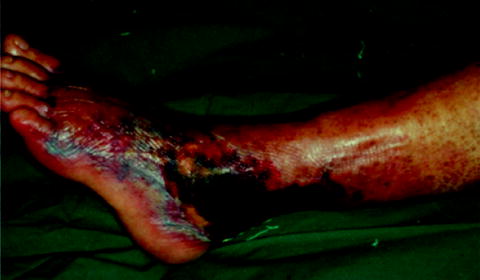

Fig. 37.2
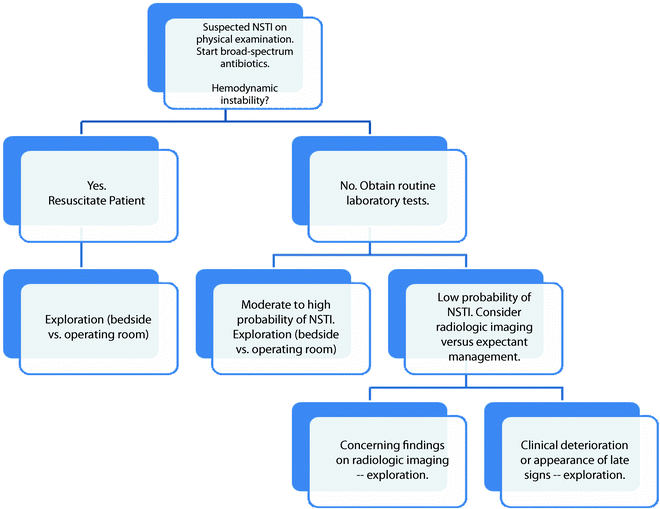
(a) This patient has minimal skin manifestations of NSTI other than erythema and swelling, characteristic of Stage I or early NSTI as proposed by Wang et al. [44] (b) The same patient after debridement of necrotic infected tissue

Fig. 37.3
This patient has multiple blisters filled with serous fluid, characteristic of Stage II

Fig. 37.4
This patient had skin necrosis and crepitus characteristic of Stage III. (Reprinted with permission from Wang YS, Wong CH, Tay YK. Staging of necrotizing fasciitis based on the evolving cutaneous features. Int J Dermatol. Oct 2007;46(10):1036–1041.)
Stage | Time course | Symptoms and signs |
|---|---|---|
Stage I | Early | Tenderness to palpation (extending beyond the apparent area of skin involvement) |
Erythema | ||
Swelling | ||
Warmth | ||
Stage II | Intermediate | Blister or bullae formation (serous fluid) |
Stage III | Late | Crepitus |
Skin anesthesia | ||
Skin necrosis with dusky discoloration |
Diagnosis
Multiple studies have demonstrated an association between a delay in diagnosis and worsened outcome from NSTIs [41–43]. The diagnosis may be obvious in the setting of the hard signs described above such as hemodynamic instability and late skin manifestations [46]. However, these findings are only present in a small percentage of NSTI patients; in a matched case-control series, necrotic skin and hypotension each occurred in only 5% of patients and no patients had crepitance [47]. Furthermore, as described previously, by the time bullae, crepitus, or skin necrosis are apparent on physical examination, the NSTI has already progressed to an intermediate or late stage [44].
Compounding the difficulties in diagnosis are the similarities in presentation between early stage NSTIs and cellulitis such as fever, pain, swelling, tenderness, erythema, and warmth [47]. In a matched case-control study, Wall et al. compared physical examination findings, laboratory values, and radiologic findings in patients with necrotizing fasciitis to those with a non-necrotizing soft tissue infection. They found that the parameters with the highest sensitivity for necrotizing fasciitis were white blood cell count greater than 14 × 109/L, sodium less than 135 mmol/L, and blood urea nitrogen greater than 15 mg/dL [47]. The parameters with the highest specificity (100% for all) were tense edema, bullae, sodium less than 135 mmol/L, and chloride less than 95 mmol/L [47]. Based on these findings, Wall et al. developed a simple model to assist in diagnosing NSTIs. A corrected serum sodium (for glucose) of less than 135 mmol/L or a white blood cell count of greater than 14.3 × 109/L had a 90% sensitivity and a 76% specificity for necrotizing fasciitis [46]. This model correctly classified 18/19 (95%) of patients who had no “hard signs” [46].
Another commonly used model for diagnosing an NSTI is the Laboratory Risk Indicator for NECrotizing fasciitis (LRINEC) score [48]. Six laboratory parameters are included in the score and are weighted from 1 to 4 points for a total possible score of 13 (Table 37.2). The probability of necrotizing infections was less than 50% with a cutoff score of less than or equal to 5, but increased to greater than 75% with a cutoff score of greater than or equal to 8 [48]. A cutoff score of 6 had a positive predictive value (PPV) of 92% and a negative predictive value (NPV) of 96% in the original validation dataset [48]. The LRINEC score has not been validated across other patient populations and settings [49, 50], although one study suggested that it may function as both a diagnostic and prognostic tool [51]. Thus, the LRINEC score may be useful in select patient populations in increasing the suspicion for a necrotizing infection, but further studies are required. As with all diagnostic tools, the predictive values are dependent on the incidence of the disease in the population, and the utility of a test in changing management depends on the level of suspicion for the disease (or the pretest probability).
Table 37.2
Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score; [48] a cutoff 6 points had a 92% positive predictive value and a 96% negative predictive value
Variable, units | Score |
|---|---|
C-reactive protein, mg/dL | |
<150 | 0 |
≥150 | 4 |
Total white cell count, per mm 3 | |
<15 | 0 |
15–25 | 1 |
>25 | 2 |
Hemoglobin, g/dL | |
>13.5 | 0 |
11–13.5 | 1 |
<11 | 2 |
Sodium, mmol/L | |
≥135 | 0 |
<135 | 2 |
Creatinine, μmol/L | |
≤141 | 0 |
>141 | 2 |
Glucose, mmol/L | |
≤10 | 0 |
>10 | 1 |
Radiographic imaging may be helpful. In the case-control study by Wall et al., 39% of patients with necrotizing fasciitis had gas on plain film versus 5% of patients with a non-necrotizing infection [47]. However, gas on X-ray only had a sensitivity of 39%. Ultrasonography has been used in a few case reports and case series as an aid in the diagnosis of NSTIs [52–54]. Ultrasound can be performed rapidly at the bedside unlike other imaging modalities such as computed tomography (CT) scans and magnetic resonance imaging (MRI). Findings of increased echogenicity of the subcutaneous tissue may be seen in NSTIs but can also be present in cellulitis. Fluid greater than 4 mm in thickness or tracking along the deep fascia may be more suggestive of an NSTI [54]. Currently, however, there is insufficient evidence to recommend routine use of ultrasound in the diagnosis of NSTIs.
Traditionally, although CT and MRI have been reported to be useful adjuncts in the diagnosis of NSTIs, there has been a hesitation to recommend their routine use due to potential delays in obtaining the studies. However, as technology continues to evolve, these studies may become more useful. Findings on CT scans consistent with NSTIs have included subcutaneous air, fascial edema and thickening, non-enhancement of necrotic tissues, gas across tissue planes, or fluid collections [55–57]. In a study of 67 patients without indication for immediate surgical exploration for NSTI, CT scans had 100% sensitivity and 81% specificity for diagnosing NSTIs [57]. Three out of eight patients with a false-positive CT scan had fluid collections identified that ultimately were diagnosed as abscesses associated with pyomyositis [57]. Another study by McGillicuddy et al. reported that 305/715 (43%) of NSTI patients diagnosed over a 10-year period at a single center underwent CT scan. They developed a scoring system of five CT findings to aid in the diagnosis of NSTIs (Table 37.3). A score of greater than 6 had 86% sensitivity, 92% specificity, 64% PPV, and 86% NPV [58]. Further prospective validation studies are planned.
Table 37.3
Computed tomography (CT) NSTI Scoring System: [58] a score of >6 points had an 86% sensitivity and a 92% specificity for the diagnosis of NSTI
Variable | Points |
|---|---|
Fascial air | 5 |
Muscle/fascial edema | 4 |
Fluid tracking | 3 |
Lymphadenopathy | 2 |
Subcutaneous edema | 1 |
MRI has been used to diagnose NSTIs, but like CT has a high sensitivity but a low specificity [3]. Findings on T2-weighted images have included: gas or low signal intensity in the deep fascia, [59, 60] abnormal deep fascial thickening with or without contrast enhancement [59, 61, 62], peripheral high signal intensity in muscles [59, 63], extensive involvement of the deep fascia [59], and involvement of three or more compartments in one extremity [59]. Concerns about availability, potential delay in diagnosis and subsequent intervention, and lack of well-defined criteria for distinguishing NSTIs from non-necrotizing infections still limit the widespread use of MRIs in establishing the diagnosis.
Fluid and tissue sampling have also been suggested for diagnosing NSTIs. A 22-gauge needle with a 10-mL syringe has been used to aspirate fluid in the setting of soft tissue infections [64]. In a study of 50 patients in whom aspiration biopsy was performed, cultures were positive in 81% of patients not on antimicrobial therapy, but the percentage dropped to 30% in patients receiving antimicrobial treatment. Growth of an organism on aspirate was not specific as the cultures were taken from patients with cellulitis, ulcers, chronic osteomyelitis, and infected surgical wounds. Furthermore, although the organisms on aspirate were similar to those in surgical specimens among patients who were subsequently debrided, there was often a delay to growth of an organism in the aspiration fluid (up to 72 h) [64]. There is inadequate evidence to recommend the routine use of aspiration biopsy to diagnose NSTIs.
Ultimately, the diagnosis of a NSTI is confirmed by surgical exploration, either at the bedside (if the patient is clinically unstable) or in the operating room. Typical gross findings include loss of tissue resistance to blunt dissection, thrombosis of subcutaneous vessels, presence of foul-smelling and/or dishwater fluid, and grayish appearance of fascia with or without obvious tissue necrosis. These findings are sufficient to confirm the diagnosis, but if the surgeon is still uncertain, frozen-section biopsy can be performed. Frozen-section biopsy for rapid and early diagnosis of necrotizing fasciitis was advocated by Stamenkovic and Lew in 1984 [65]. They recommended obtaining at least a 10 × 7 × 7 mm incisional biopsy of soft tissue under local anesthetic. Their criteria for the histologic diagnosis are listed in Table 37.4. In their small case series, which included eight subsequently confirmed cases of necrotizing fasciitis, the histology revealed intact superficial epidermis and dermis and a combination of edema, vasculitis and thrombosis, neutrophilic infiltration, and microorganisms in the deeper layers including deep dermis, subcutaneous fat, and fascia [65]. Histologic samples from patients who did not undergo frozen section biopsy demonstrated further extension of the necrosis representative of progressive disease. Use of frozen section biopsy, however, is limited by the availability of a pathologist to read the samples, and necrotizing infections are usually associated with obvious findings such as those described previously.
Necrosis of superficial fascia |
Polymorphonuclear infiltration of the deep dermis and fascia |
Fibrinous thrombi of vessels passing through the fascia |
Angiitis with fibrinoid necrosis of vessel walls |
Presence of microorganisms within the destroyed tissue on Gram stain |
No muscle involvement |
Management
The mainstay of treatment for NSTIs is administration of broad spectrum antibiotics and prompt and aggressive surgical debridement of infected tissues (Fig. 37.5). Randomized trials of adjunctive treatments are lacking, and synthesis of observational studies is hampered by: (1) a lack of standardized terminology and (2) heterogeneity in patient populations, bacteriology, and management strategies.