83 Myocarditis and Acute Myopathies
 Myocarditis in the Intensive Care Unit
Myocarditis in the Intensive Care Unit
Myocarditis is defined as inflammation of heart muscle.1 Many different etiologic agents have been implicated in this disease, but viral infections are the most common cause. Myocarditis is also associated with autoimmune and other systemic diseases.2 The clinical picture of myocarditis varies widely, from asymptomatic patients who recover without specific therapy and suffer no long-term sequelae to critically ill patients with heart failure and cardiogenic shock. There are no standardized, specific, and widely agreed-upon criteria for making the diagnosis of myocarditis or for determining a cause in many patients.3 Lastly, there has been controversy regarding the most appropriate medical therapy for this condition.
On pathologic examination of myocardial biopsy specimens or on autopsy series, myocarditis is usually apparent as infiltration of myocardium with lymphocytes and fibroblasts, accompanied by myocyte necrosis (myocytolysis).3 It is this type of myocarditis, often termed lymphocytic myocarditis, that will be referred to in this chapter unless otherwise specified. Other types of inflammatory reactions can be seen less frequently in myocarditis, involving giant cells, eosinophils, or granulomas, which can be associated with specific clinical conditions.
In most patients with myocarditis, a specific cause is not found.4 It is presumed that in North America and Europe, the most common etiologic agent is viral.1 Coxsackie B enterovirus was felt to be the most common cause up to the 1990s, but adenoviruses and parvovirus 19 have been implicated as causative agents more frequently over the past 20 years. Other viral causes include hepatitis C, cytomegalovirus, and human herpesvirus 6.2 Myocarditis is a common finding in patients infected with human immunodeficiency virus (HIV). However, the causative agent responsible in these cases may be a secondary viral infection such as cytomegalovirus or other opportunistic infection such as mycobacteria, fungi, or parasites, rather than HIV itself.1,5,6 Infectious illnesses such as Lyme disease, acute rheumatic fever, and diphtheria often have myocarditis as a prominent feature. In Central and South America, the most common cause of myocarditis is the protozoan, Trypanosoma cruzi, the cause of Chagas’ disease (Table 83-1). Systemic and autoimmune diseases such as systemic lupus erythematosus, polymyositis, scleroderma, sprue, Whipple’s disease, and sarcoidosis can be complicated by myocarditis, and myocarditis can be a feature of the infiltrative cardiomyopathies seen in hemochromatosis or amyloidosis. Idiopathic specific forms of myocarditis include hypersensitivity or eosinophilic myocarditis, which has also been reported after smallpox vaccination,7 and giant cell myocarditis.8 Lastly, myocarditis can be associated with doxorubicin cardiomyopathy or with peripartum cardiomyopathy, or it can be a manifestation of a hypersensitivity reaction to medications9,10 (Table 83-2).
TABLE 83-1 Causes of Myocarditis*
| Infectious | Immune-Mediated | Toxic Myocarditis |
|---|---|---|
| Bacterial: Brucella, Corynebacterium diphtheriae, gonococcus, Haemophilus influenzae, meningococcus, Mycobacterium, Mycoplasma pneumoniae, pneumococcus, salmonella, Serratia marcescens, staphylococcus, Streptococcus pneumoniae, Streptococcus pyogenes, Treponema pallidum, Tropheryma whippelii, and Vibrio cholerae Spirochetal: Borrelia and Leptospira Fungal: actinomyces, aspergillus, blastomyces, Candida, Coccidioides, Cryptococcus, Histoplasma, mucormycoses, Nocardia, and Sporothrix Protozoal: Toxoplasma gondii and Trypanosoma cruzi Parasitic: ascaris, Echinococcus granulosus, Paragonimus westermani, Schistosoma, Taenia solium, Trichinella spiralis, visceral larva migrans, and Wuchereria bancrofti Rickettsial: Coxiella burnetii, Rickettsia rickettsii, and Rickettsia tsutsugamushi Viral: coxsackievirus, cytomegalovirus, dengue virus, echovirus, encephalomyocarditis, Epstein-Barr virus, hepatitis A virus, hepatitis C virus, herpes simplex virus, herpes zoster, human immunodeficiency virus, influenza A virus, influenza B virus, Junin virus, lymphocytic choriomeningitis, measles virus, mumps virus, parvovirus, poliovirus, rabies virus, respiratory syncytial virus, rubella virus, rubeola, vaccinia virus, varicella-zoster virus, variola virus, and yellow fever virus | Allergens: acetazolamide, amitriptyline, cefaclor, colchicine, furosemide, isoniazid, lidocaine, methyldopa, penicillin, phenylbutazone, phenytoin, reserpine, streptomycin, tetanus toxoid, tetracycline, and thiazides Alloantigens: heart transplant rejection Autoantigens: Chagas’ disease, Chlamydia pneumoniae, Churg-Strauss syndrome, inflammatory bowel disease, giant cell myocarditis, insulin-dependent diabetes mellitus, Kawasaki’s disease, myasthenia gravis, polymyositis, sarcoidosis, scleroderma, systemic lupus erythematosus, thyrotoxicosis, and Wegener’s granulomatosis | Drugs: amphetamines, anthracyclines, catecholamines, cocaine, cyclophosphamide, ethanol, fluorouracil, hematin, interleukin-2, lithium, and trastuzumab Heavy metals: copper, iron, and lead Physical agents: electric shock, hyperpyrexia, and radiation Miscellaneous: arsenic, azides, bee and wasp stings, carbon monoxide, inhalants, phosphorus, scorpion bites, snake bites, and spider bites |
* The most common causes are shown in boldface type.
From Feldman A, McNamara D. Myocarditis. N Engl J Med 2000;343:1388-98.
TABLE 83-2 Distinct Forms of Myocarditis
From Haas G. Etiology, evaluation, and management of acute myocarditis. Cardiol Rev 2001;9:88-95.
Unfortunately, it is difficult to make a clinical diagnosis of a specific viral cause of myocarditis. This usually requires measurement of antiviral antibody titers in acute and convalescent-phase sera. Viral cultures of tissue specimens are unreliable.4 Identification of viral genomes incorporated in myocyte DNA suggests but does not specifically prove that the virus is the cause.
 Pathogenesis
Pathogenesis
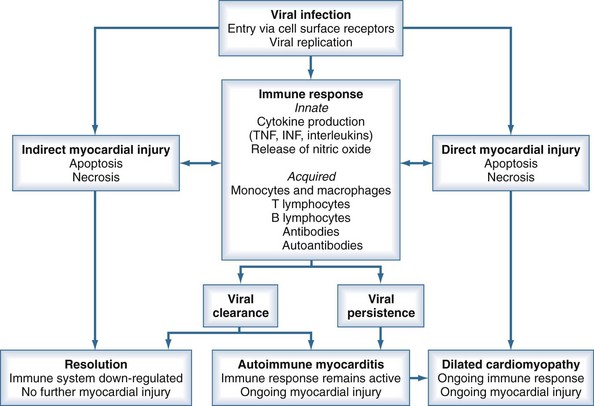
Based on observations of human myocarditis, as well as murine models of the disease caused by coxsackie B3, the pathogenesis of viral myocarditis can be described in three stages.2,11 The first stage is initiated by viral infection and replication within myocytes. Viral proteases and activation of cytokines may produce myocyte damage and apoptosis.12 The presence of this viral replication phase is difficult to detect clinically because patients may be asymptomatic during this phase or only have nonspecific viremic symptoms. In addition, there is no rapid screening test to confirm viral infection.
The second stage involves host immune activation. Stimulation of cellular immunity and humoral responses attenuates viral proliferation and can result in recovery from the illness. However, unabated immune activation can result in activated T cells targeting myocardial antigens that cross-react with viral peptides. This leads to release of cytokines such as tumor necrosis factor (TNF), interleukin (IL)-1, and IL-6, resulting in further myocyte damage.1,12 Activation of CD4 cells and antibody production plays a less important pathogenetic role. It is believed that this secondary immune response to viral infection plays a greater role in disease pathogenesis than the primary infection.12
Evidence supporting these mechanisms includes several key observations. Myocardial biopsy with recombinant DNA techniques can detect viral genomes in 20% to 35% of patients. Tissue-specific autoantibodies have been detected in 25% to 73% of patients with evidence of myocarditis on biopsy, with antibodies directed against contractile, structural, and mitochondrial myocyte proteins. Inappropriate expression of the major histocompatibility complex can frequently be demonstrated on biopsy specimens.1 Elevated levels of inflammatory cytokines are detected in patients with active myocarditis.
Either persistent overactivation of cellular immune activity or incomplete clearing with persistent or recurrent viral replication can lead to the third stage, during which significant myocardial damage occurs. This leads to left ventricular (LV) dilatation and remodeling, LV systolic dysfunction, and manifestations of heart failure.12 These processes can then abate, with reduction in LV size and improvement of LV function, or can continue to progress with development of dilated cardiomyopathy, worsening ventricular function, and chronic heart failure. Chronic dilated cardiomyopathy is the major long-term sequela of acute myocarditis (Figure 83-1).
 Clinical Presentation and Diagnosis
Clinical Presentation and Diagnosis
The incidence of myocarditis is difficult to determine; many cases are mild with subclinical disease. Myocarditis is diagnosed on clinical grounds, as there are no specific clinical diagnostic criteria. The presentation of myocarditis varies widely. Patients can be asymptomatic insofar as myocarditis has been found in 1% to 10% of autopsy specimens of young adults who had no history of cardiac illness. Myocarditis can be found at autopsy in up to 20% of cases of young, apparently healthy adults who die suddenly and unexpectedly.1,4,10
Patients ill with myocarditis present with nonspecific symptoms of dyspnea (72%), chest pain (32%), and symptoms of arrhythmia (18%).13 The presentation may be indistinguishable from acute coronary syndromes due to coronary artery disease. There may have been a preceding viral prodrome with fever, malaise, and arthralgias. Physical examination can show fever, tachycardia, S3 and S4 gallop sounds, and a pericardial rub if myopericarditis is present. Signs of heart failure can be present, including pulmonary rales and wheezes, elevated jugular venous pulse, and peripheral edema. Murmurs of mitral regurgitation and tricuspid regurgitation may be heard. Infrequently, the presentation is fulminant and severe, with acute heart failure, pulmonary edema, and cardiogenic shock.4
Laboratory findings can include leukocytosis, eosinophilia, and an elevated erythrocyte sedimentation rate. Cardiac biomarkers such as creatine kinase, troponin T, and troponin I may be elevated, with sensitivity of troponin I reported at 34% and specificity of 89%.14 Rheumatologic serologic markers and HIV status should be evaluated.
The 12-lead electrocardiogram (ECG) is an insensitive test for the diagnosis of myocarditis. It shows sinus tachycardia and nonspecific ST-segment depression and T-wave inversion most often. Patients may present with chest pain and ST-segment elevation, with a picture mimicking AMI. More severe cases can be associated with supraventricular or ventricular arrhythmias, conduction disturbances, and heart block.1
Echocardiography is essential to diagnose and quantitate regional or global LV wall-motion abnormalities, left ventricular and right ventricular size and function, the presence of pericardial effusion, and valvular regurgitation. Fulminant myocarditis is characterized by a nondilated left ventricle, with severe systolic dysfunction and increased wall thickness reflecting myocardial edema.15 Findings on myocardial nuclear scintigraphy are frequently abnormal, but this test is not useful in the diagnosis of myocarditis. Cardiac catheterization and coronary angiography are often necessary to exclude acute ischemia as the cause of chest pain or acute heart failure.
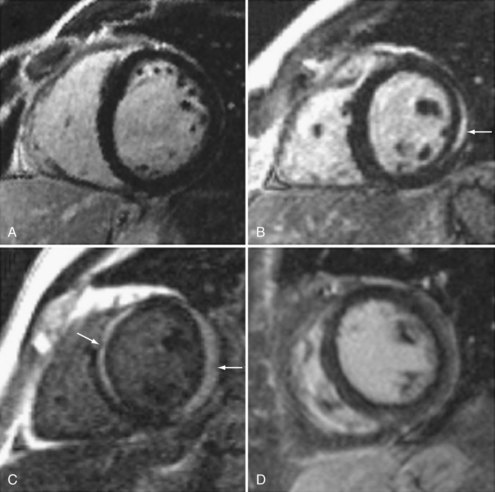
There is increasing use of cardiac magnetic resonance imaging (CMR) in the diagnosis of myocarditis.16,17,18 This technique has the potential to offer a noninvasive means to make this diagnosis. CMR should be considered in symptomatic patients with a high clinical suspicion of disease when the results are likely to affect management decisions. Diagnostic criteria include: (1) focal or diffuse myocardial edema in T2-weighted images, (2) early gadolinium enhancement indicating inflammation, and (3) late gadolinium enhancement in subepicardial or mid-myocardial areas indicating necrosis and fibrosis. Abnormalities may be diffuse or patchy, often confined to the lateral free wall of the left ventricle or the base of the interventricular septum (Figure 83-2). Diagnostic accuracy of CMR is reported at 78% when 2 or 3 criteria are present and 68% when only late gadolinium enhancement is present. CMR is more likely to be abnormal when performed more than 7 days after onset of symptoms. CMR may also detect pericardial effusion (seen in 32%-57% of patients) and gives information regarding LV function. CMR can also be used to direct myocardial biopsy in patients with patchy uptake. The value of CMR for assessing prognosis is unknown, and this presently represents a major limitation of this diagnostic technique.19,20,21
 Endomyocardial Biopsy
Endomyocardial Biopsy
Percutaneous endomyocardial biopsy (EMB) is currently used to aid in the diagnosis of myocarditis and is considered the definitive diagnostic technique. The Dallas criteria have been accepted as the standard for histopathologic diagnosis. These criteria define active myocarditis as the presence of an inflammatory myocardial infiltrate (more than five lymphocytes per high-power field) accompanied by myocyte necrosis. Borderline myocarditis is defined as inflammation without myocyte necrosis. However, there is no difference in prognosis in patients with either of these biopsy results.9 Thus, lymphocyte infiltration (with or without myocyte necrosis) is the most important diagnostic criterion.
Although EMB is useful for diagnostic purposes, there are a number of significant limitations. A high frequency of interobserver variation has been noted among pathologists in applying the Dallas criteria. Biopsies are not sensitive in diagnosing myocarditis; various series have reported positive right ventricular biopsy results in only 10% to 67% of patients with myocarditis suspected on clinical grounds or with recent-onset idiopathic dilated cardiomyopathy. This variability may relate to the timing of biopsies in respect to the stage or chronicity of the patient’s illness. In addition, the myocardial inflammation may not be diffuse and may be patchy, or may predominantly involve the left ventricle, so random right ventricular biopsies may miss affected myocardium.22 Thus, performing a biopsy earlier in a patient’s clinical course, taking multiple biopsy specimens, and performing LV biopsies are ways of improving diagnostic yield. In addition, immunohistochemical staining for human leukocyte antigens can improve diagnostic sensitivity.11,23 EMB should be performed in centers with a high-volume experience, with proven safety and availability of appropriate pathologic techniques.24 However, it is important to emphasize that a negative biopsy finding does not preclude the diagnosis of myocarditis.
Although EMB is an insensitive test with a number of problems, a positive biopsy finding has a high positive predictive value.9 Some authors question the benefits of performing biopsy with standard staining techniques as a routine in suspected myocarditis cases, but this remains the best diagnostic test currently available. Other analyses such as examining specimens for viral genomes utilizing polymerase chain reaction (PCR) or using immunohistochemistry technology to identify up-regulated HLA proteins may offer improved diagnostic yield.22
Endomyocardial biopsy should be strongly considered in cases of suspected myocarditis when pathology results will affect management decisions. A recent American Heart Association/American College of Cardiology/European Society of Cardiology (AHA/ACC/ESC) scientific statement offered recommendations concerning the appropriate use of EMB based on patients’ clinical presentations.25 EMB was deemed useful, beneficial. and effective (class I indication) in patients with acute heart failure with hemodynamic compromise, after causes such as coronary artery disease are excluded. EMB is this setting is necessary to differentiate giant cell myocarditis and eosinophilic myocarditis from lymphocytic myocarditis, since immunosuppressive therapy is mandated in the first two conditions (see later). A class I indication for EMB was also recommended for patients with new-onset subacute heart failure, with duration of illness of 2 weeks to 3 months, who fail to improve with medical therapy for heart failure or who demonstrate severe ventricular arrhythmia or advanced heart block. EMB should be considered if causes such as sarcoidosis or collagen vascular disease are suspected and should be performed to diagnose giant cell myocarditis or eosinophilic myocarditis.26 Endomyocardial biopsy should always be performed prior to initiating immunosuppressive therapy (Table 83-3).
TABLE 83-3 Indications for Endomyocardial Biopsy
Adapted with permission from Wu L, Lapeyre A, Cooper L. Current role of endomyocardial biopsy in the management of dilated cardiomyopathy and myocarditis. Mayo Clin Proc 2001;76:1030-8.
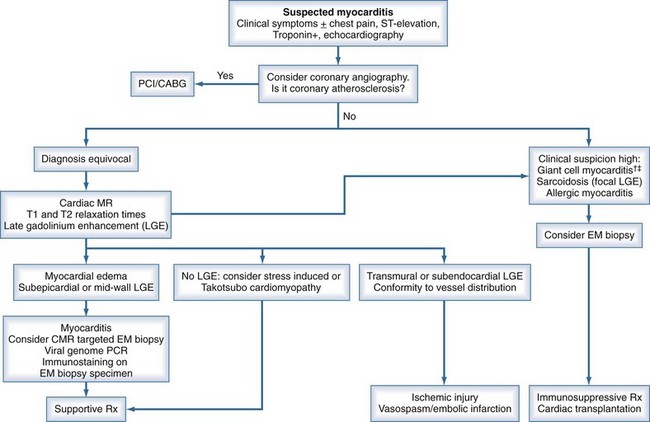
An algorithm has been proposed outlining the steps in evaluating patients suspected of having acute myocarditis (Figure 83-3).
 Clinical Course and Prognosis
Clinical Course and Prognosis
The clinical course and prognosis of acute myocarditis is variable. The majority of patients diagnosed with myocarditis will improve. Patients with mild symptoms most often recover without complications. Eight to 12% of young, apparently healthy adults who die suddenly from a cardiac cause are found to have myocarditis at autopsy, suggesting that patients even with apparently mild illness can suffer fatal arrhythmias.11 Some patients with myocarditis will progress to chronic dilated cardiomyopathy with manifestations of systolic heart failure,3 although a precise incidence is not known. Fifteen to 25% of patients who present with new-onset dilated cardiomyopathy have evidence for antecedent myocarditis.3 Patients with heart failure and LV dysfunction will experience spontaneous resolution of their illness within 12 months in up to 40% of cases, without long-term sequelae. Roughly one-quarter of patients with acute myocarditis and ejection fraction less than 35% will improve, half will develop chronic cardiomyopathy and heart failure, and one-quarter will deteriorate and may be candidates for cardiac transplantation.27
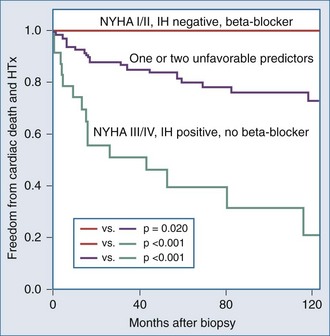
It is important to examine the patient population under study and the criteria used for diagnosing myocarditis in any series assessing prognosis and mortality. No clinical markers reliably predict which patients with myocarditis will recover or worsen.9 In the Myocarditis Treatment Trial, 1-year mortality rate was 20% and 5-year mortality was 56% in patients with biopsy-confirmed lymphocytic myocarditis.28 A series of 21 patients with active myocarditis on biopsy was analyzed for predictors of disease course. Variables assessed included baseline hemodynamics, use of ventilatory and circulatory support, and serum cardiac biomarkers. Overall, there was a 37% mortality rate (8 of 21), with death occurring at 27.6 ± 6.9 days. Factors predicting a worse prognosis included hypotension (mean 84/49 mm Hg), higher pulmonary capillary wedge pressure (mean of 24 mm Hg), and use of mechanical ventilation. Factors that were not predictive of mortality included sex, age, heart rate, cardiac index, peak creatine kinase, or the use of intraaortic balloon counterpulsation for circulatory support.29 Another trial reported 181 patients with myocarditis confirmed by EMB utilizing the Dallas criteria, immunohistochemical staining and PCR, which assesses for viral genome. LV biopsy was performed in 90% of patients. Patients were followed for an average of 59 months, and 22% died or received cardiac transplantation. Multivariate analysis concluded that functional class III and IV heart failure and a positive immunohistochemical result were the only predictors of poor outcome, and treatment with beta-blockers was associated with better outcomes23 (Figure 83-4). Other series have reported that LV ejection fraction (LVEF) less than 40% and right ventricular dysfunction also predict a poorer prognosis.11
Fulminant Myocarditis
In a study of 147 patients presenting with heart failure due to biopsy-positive active myocarditis with ejection fraction less than 40%, 10% of patients were diagnosed with fulminant myocarditis and 90% with acute lymphocytic myocarditis.8 The patients with fulminant myocarditis needed hemodynamic support with high-dose vasopressors or left ventricular assist devices (LVADs). The acute myocarditis patients had more stable hemodynamics and did not require vasopressors or received them at low doses. Patients with fulminant myocarditis tended to be younger and had higher heart rates and lower systemic blood pressure. There was no difference between the groups in mean pulmonary capillary wedge pressure or cardiac index.
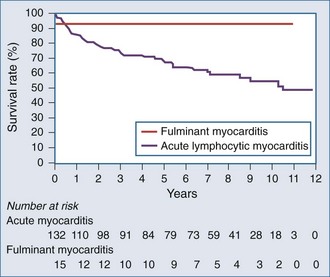
In summary, fulminant myocarditis has a distinct clinical course, with critical illness at presentation but with excellent long-term survival once patients recover from the acute phase of their illness. Healing of myocardial injury and significant improvement of LV systolic function can be expected. Therefore, an aggressive approach to therapy, including the use of ventricular assist devices or other mechanical assist devices, without resorting to early cardiac transplantation, is warranted (Figure 83-5).9
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree