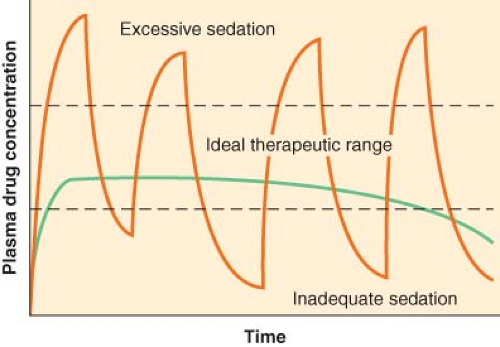
Monitored Anesthesia Care
Simon C. Hillier
Michael S. Mazurek
Jeana E. Havidich
Key Points
Related Matter
Continuous Infusion
During monitored anesthesia care, the continuous attention of the anesthesiologist is directed at optimizing patient comfort and safety. Monitored anesthesia care usually involves the administration of drugs with anxiolytic, hypnotic, analgesic, and amnestic properties, either alone or as a supplement to a local or regional technique.
Terminology
Conceptually, monitored anesthesia care is attractive because it should invoke less physiologic disturbance and allow a more rapid recovery than general anesthesia. It is instructive to review the ASA position statement that defines monitored anesthesia care:3
Monitored anesthesia care is a specific anesthesia service for a diagnostic or therapeutic procedure. Indications for monitored anesthesia care include the nature of the procedure, the patient’s clinical condition, and/or the potential need to convert to a general or regional anesthetic.
Monitored anesthesia care includes all aspects of anesthesia care—a preprocedure visit, intraprocedure care, and postprocedure anesthesia management. During monitored anesthesia care, the anesthesiologist provides or medically directs a number of specific services, including but not limited to:
Diagnosis and treatment of clinical problems that occur during the procedure
Support of vital functions
Administration of sedatives, analgesics, hypnotics, anesthetic agents, or other medications as necessary for patient safety
Psychological support and physical comfort
Provision of other medical services as needed to complete the procedure safely.
Monitored anesthesia care may include varying levels of sedation, analgesia, and anxiolysis as necessary. The provider of monitored anesthesia care must be prepared and qualified to convert to general anesthesia when necessary. If the patient loses consciousness and the ability to respond purposefully, the anesthesia care is a general anesthetic, irrespective of whether airway instrumentation is required.
Monitored anesthesia care is a physician service provided to an individual patient. It should be subject to the same level of payment as general or regional anesthesia. Accordingly, the ASA Relative Value Guide provides for the use of proper base procedural units, time units, and modifier units as the basis for determining payment.
The ASA also states that monitored anesthesia care should be requested by the attending physician and be made known to the patient, in accordance with accepted procedures of the institution. In addition, the ASA states that the service must include the following:
Performance of a preanesthetic examination and evaluation.
Prescription of anesthetic care.
Personal participation in, or medical direction of, the entire plan of care.
Continuous physical presence of the anesthesiologist or, in the case of medical direction, of the resident or nurse anesthetist being medically directed.
Proximate presence, or in the case of medical direction, availability of the anesthesiologist for diagnosis and treatment of emergencies.
Furthermore, the ASA states that all institutional regulations pertaining to anesthesia services shall be observed, and all the usual services performed by the anesthesiologist shall be furnished, including but not limited to:
Usual noninvasive cardiocirculatory and respiratory monitoring.
Oxygen administration, when indicated.
Administration of sedatives, tranquilizers, antiemetics, narcotics, other analgesics, beta-blockers, vasopressors, bronchodilators, antihypertensives, or other pharmacologic therapy as may be required in the judgment of the anesthesiologist.
Preoperative Assessment
The preoperative evaluation is an essential prerequisite to monitored anesthesia care and should be as comprehensive as that performed prior to any general or regional anesthetic (see Chapter 22). However, in addition to the usual evaluation for the patient who is scheduled to undergo general anesthesia, there are additional considerations unique to the monitored anesthesia care that may ultimately determine the success or failure of the procedure. It is important to evaluate the patient’s ability to remain motionless and, if necessary, actively cooperate throughout the procedure. Thus, it is important to evaluate the patient’s psychological preparation for the planned procedure. It is also important to elicit the presence of coexisting sensorineural or cognitive deficits. These factors or the inability to communicate with the patient may occasionally make general anesthesia a more appropriate alternative. Verbal communication between physician and patient is very important for three reasons: (1) as a monitor of the level of sedation and cardiorespiratory function, (2) as a means of explanation and reassurance for the patient, and (3) as a mechanism of communication when the patient is required to actively cooperate. Although cardiorespiratory disease is often cited as an indication to perform a procedure using monitored anesthesia care rather than general anesthesia, there are occasions when cardiorespiratory disease may reduce the utility of monitored anesthesia care. For example, the presence of a persistent cough may make it very difficult for the patient to remain immobile, which can be particularly dangerous during ophthalmologic or awake neurosurgical procedures. Attempts to attenuate coughing with sedation techniques are likely to be unsuccessful and potentially harmful because a significant level of anesthesia is required to abolish the cough reflex. Similarly, some patients with significant cardiovascular or pulmonary disease may be unable to lie flat for an extended period.
Techniques of Monitored Anesthesia Care
A variety of medications are commonly administered during monitored anesthesia care with the desired end points to provide patient comfort, maintain cardiorespiratory stability, improve operating conditions, and prevent recall of unpleasant perioperative events. It is helpful to delineate and individualize the goals for each patient in order to formulate an appropriate regimen, which frequently involves the administration of either individual or combinations of analgesic, amnestic, and hypnotic drugs. There should be a minimal incidence of side effects, such as cardiorespiratory depression, nausea and vomiting, delayed emergence, and dysphoria, and there should be a rapid and complete recovery. Ideally, the patient should be able to communicate during the procedure. Clinical experience suggests that a level of sedation that allows verbal communication is optimal for the patient’s comfort and safety. If the level of sedation is deepened to the extent that verbal communication is lost, the risks of the technique approach those of general anesthesia with an unprotected and uncontrolled airway. However, because monitored anesthesia care is provided by anesthesiologists, the range of sedation may include deeper sedation techniques than those provided by nonanesthesiologists during sedation/analgesia.
The preanesthetic evaluation and plan should identify specific causes of and provide specific therapy for pain, anxiety, and agitation. Pain may be treated by local or regional analgesia, systemic analgesics, or removal of the painful stimulus. Anxiety may be reduced by the use of an anxiolytic such as a benzodiazepine and reassurance by the anesthesiologist. Patient agitation may be a result of pain or anxiety or life-threatening factors such as hypoxia, hypercarbia, impending local anesthetic toxicity, and cerebral hypoperfusion. Other causes of pain and agitation include a distended bladder, hypothermia, hyperthermia, pruritus, nausea, positional discomfort, uncomfortable oxygen masks and nasal cannulae, intravenous (IV) cannulation site infiltration, a member of the surgical team leaning on the patient, and prolonged pneumatic tourniquet inflation.
Pharmacologic Basis of Monitored Anesthesia Care Techniques—Optimizing Drug Administration
The ability to predict the effects of the drugs in our armamentarium demands an understanding of their pharmacokinetic and pharmacodynamic properties. This understanding is a fundamental prerequisite for the design of an effective sedation regimen and greatly increases the probability of producing the desired therapeutic effect. Context-sensitive half-time, effect–site equilibration time, and anesthetic/sedative drug interactions are fundamental concepts that are particularly useful in the context of monitored anesthesia care and will be discussed in some detail.
Distribution, Elimination, Accumulation, and Duration of Action
Following the administration of IV anesthetic drugs, the immediate distribution phase causes a brisk decrease in plasma levels as the drug is transported to the rapidly equilibrating vessel-rich group of tissues. There is a simultaneously occurring distribution of drug to the less well-perfused tissues such as muscle and skin. Over time, the drug is also distributed to the poorly perfused tissues such as bone and fat. Although the latter compartments are poorly perfused, they may accumulate significant amounts of lipophilic drugs during prolonged administration. This peripheral depot may contribute to a delayed recovery when the drug is eventually released back into the central compartment after its
administration is discontinued. Redistributive factors are important determinants of drug effect and influence the plasma concentration of a drug in a time-dependent fashion.
administration is discontinued. Redistributive factors are important determinants of drug effect and influence the plasma concentration of a drug in a time-dependent fashion.
The Elimination Half-life
Until recently, the elimination half-time was the predominant pharmacokinetic parameter used as the predictor of an anesthetic drug’s duration of action. In everyday clinical practice, however, this parameter has not greatly enhanced our ability to predict anesthetic drug disposition. Only in single-compartment models does the elimination half-time actually represent the time required for a drug to reach half of its initial concentration after administration. In a single-compartment model, elimination is the only process that can alter drug concentration. Intercompartmental distribution cannot occur because there are no other compartments for the drug to be distributed to and fro. Most drugs in the anesthesiologist’s armamentarium are lipophilic and are, therefore, more suited to multicompartmental modeling than single-compartment modeling. Similarly, other pharmacokinetic parameters, such as distribution half-time, distribution volume, intercompartmental rate constants, and so forth, do not provide us with a practical means of predicting drug disposition. In multicompartmental models, the metabolism and excretion of some IV anesthetic drugs may have only a minor contribution to the changes in plasma concentration when compared with the effects of intercompartmental distribution.
Context-sensitive Half-time
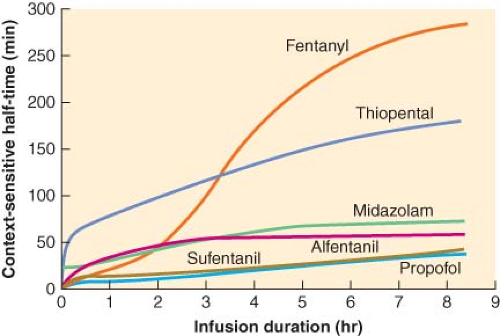 Figure 29.2. Context-sensitive half-time as a function of infusion duration. These data were generated from the computer model of Hughes et al.5 It can be seen that the context-sensitive half-time of propofol demonstrates a minimal increase as the duration of the infusion increases. Also note that for infusions of short duration, sufentanil has a shorter half-time than alfentanil. (Reproduced from Hughes MA, Glass PSA, Jacobs JR. Context-sensitive half-time in multicompartment pharmacokinetic models for intravenous anesthetic drugs. Anesthesiology 1992;76:334, with permission.) |
Alfentanil is the opioid that has, until recently, been most frequently studied, described, and promoted in the context of ambulatory techniques. Alfentanil has a very short elimination half-time, one-fifth that of sufentanil (111 vs. 577 minutes). However, despite the longer elimination half-time of sufentanil, its context-sensitive half-time is actually less than that of alfentanil for infusions up to 8 hours in duration. This phenomenon is explained in part by the huge distribution volume of sufentanil. After termination of a sufentanil infusion, the decay in plasma drug concentrations is accelerated not only by elimination but also by the continued redistribution of sufentanil into peripheral compartments. On the other hand, the small distribution volume of alfentanil equilibrates rapidly; therefore, peripheral distribution of drug away from the plasma is not a significant contributor to the decay in plasma concentration after an infusion. The data derived from computer simulation by Hughes et al.5 show that the plasma decay of alfentanil is slower than that of sufentanil following infusions of similar duration to those used during
conscious sedation. Thus, despite its short elimination half-time, alfentanil may not necessarily be superior to sufentanil.8
conscious sedation. Thus, despite its short elimination half-time, alfentanil may not necessarily be superior to sufentanil.8
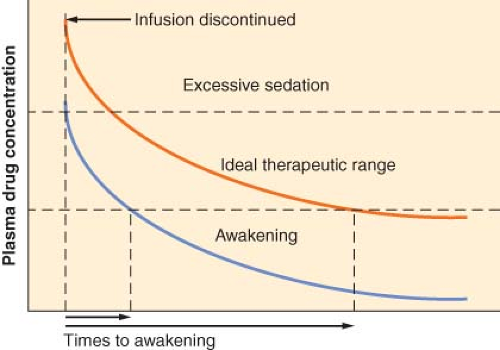
How Does the Context-sensitive Half-time Relate to the Time to Recovery?
Although the context-sensitive half-time represents a significant advance in our ability to describe drug disposition, this parameter does not directly describe how long it will take the patient to recover from monitored anesthesia care. The context-sensitive half-time merely describes how long it will take for the plasma concentration of the drug to decrease by 50%. The time to recovery depends on other additional factors. The difference between the plasma concentration at the end of the infusion and the plasma concentration below which awakening can be expected is an obvious factor in determining time to recovery. For example, if the drug concentration is maintained at a level just above that required for awakening, the time to recovery will be more rapid than after an infusion during which the drug concentration is much greater than that required for awakening (Fig. 29-3). Furthermore, although context-sensitive half-time is a reflection of plasma drug decay, awakening from anesthesia is actually a function of effect–site (i.e., brain) concentration decay. Changes in effect–site concentration demonstrate a variable time lag behind changes in plasma drug concentration. Effect–site equilibration is a concept that is particularly relevant to IV sedation. When a drug is administered intravenously by bolus or infused rapidly, there is a delay before the onset of clinical effect. This delay occurs because the plasma is not usually the site of action but is merely the route by which the drug reaches its effect site. If some parameter of drug effect can be measured (e.g., power spectrum electroencephalographic [EEG] analysis in the case of opioids), the half-time of equilibration between drug concentration in the blood and the drug effect can then be determined.9 This parameter is abbreviated t1/2ke0. Drugs with a short t1/2ke0 will equilibrate rapidly with the brain and have a shorter delay in onset than drugs that have a longer t1/2ke0. Thiopental, propofol, and alfentanil have short t1/2ke0 values compared with midazolam, sufentanil, and fentanyl.
The t1/2ke0 allows predictions to be made of the time course of equilibration of the drug between the blood and the brain. A distinct time lag between the peak serum fentanyl concentration and the peak EEG slowing can be seen. In contrast, following alfentanil administration, the EEG changes closely parallel serum concentrations. The t1/2ke0 for fentanyl is 6.4 minutes compared with a t1/2ke0 of 1.1 minutes for alfentanil. If an opioid is required to blunt the response to a single brief stimulus, alfentanil might represent a logical choice over fentanyl. The t1/2ke0 is an important determinant of bolus spacing when titrating drugs to clinical effect. In the case of drugs like midazolam and propofol, boluses of drug should be spaced far enough apart to allow the full peak effect to be clinically appreciated before further drug administration in order to avoid inadvertent overdosing.10,11,12 For example, even if the shortest quoted equilibration half-time for midazolam (0.9 minutes) is used, it will take 2.7 minutes for effect–site concentrations to be 87.5% equilibrated. Other factors are also important determinants of bolus size and spacing. For example, a low cardiac output will markedly delay drug arrival at the site of action. If sufficient time is not given for the drug to take effect before giving additional drug increments, significant cardiorespiratory compromise may occur. Furthermore, the effects of initial doses of most drugs in anesthetic practice are terminated by redistribution, which depends on blood flow to redistribution sites. If there is reduced blood flow to redistribution sites because of pre-existing and iatrogenic decreases in cardiac output, the dangerous adverse effects of these drugs are likely to be both delayed and markedly prolonged. An example of this scenario is the patient with a hemodynamic compromise caused by a tachydysrhythmia who requires sedation for cardioversion. Careful, well-spaced, small boluses of drug should be given to induce the appropriate level of sedation, bearing in mind that it may take several minutes for the full effect of a small bolus dose to become apparent.
Drug Interactions in Monitored Anesthesia Care
Drug interactions may have both a pharmacodynamic and a pharmacokinetic basis and may vary depending on the combination of drugs being coadministered, the dose range over which these drugs are administered, and the specific clinical effect that is measured. For example, because fentanyl is primarily an analgesic rather than a hypnotic, it reduces propofol requirements for suppression of response to skin incision to a much greater degree than it reduces propofol requirements for induction of anesthesia.16 On the other hand, because midazolam has significant hypnotic properties, it displays significant synergism with propofol or thiopental when used to induce hypnosis.17,18,19
The plasma concentration of a drug at steady state that is required to abolish purposeful movement at skin incision in 50%
of patients (Cpss50) is a measure of potency that is analogous to the familiar parameter of minimum alveolar concentration (MAC) of the volatile inhaled anesthetics. IV anesthetic interactions may be evaluated by their effect on the Cpss50 in a manner analogous to the expression of the effects of opioids on volatile anesthetic requirements in terms of MAC reduction.20 For example, during general anesthesia, opioid requirements to suppress the responses to noxious stimuli are tenfold higher when used as the sole agent compared with when they are used in conjunction with a nitrous oxide/potent inhaled vapor technique. This interaction persists at the lighter levels of anesthesia encountered during monitored anesthesia care. Therefore, it is likely that a rapid recovery would be facilitated by using opioids in combination with other agents (e.g., propofol/midazolam) rather than as the sole drug.
of patients (Cpss50) is a measure of potency that is analogous to the familiar parameter of minimum alveolar concentration (MAC) of the volatile inhaled anesthetics. IV anesthetic interactions may be evaluated by their effect on the Cpss50 in a manner analogous to the expression of the effects of opioids on volatile anesthetic requirements in terms of MAC reduction.20 For example, during general anesthesia, opioid requirements to suppress the responses to noxious stimuli are tenfold higher when used as the sole agent compared with when they are used in conjunction with a nitrous oxide/potent inhaled vapor technique. This interaction persists at the lighter levels of anesthesia encountered during monitored anesthesia care. Therefore, it is likely that a rapid recovery would be facilitated by using opioids in combination with other agents (e.g., propofol/midazolam) rather than as the sole drug.
Drug interactions are dose dependent. For example, when fentanyl is combined with isoflurane, the greatest reduction in isoflurane MAC occurs within the analgesic concentration range of fentanyl (i.e., 1 to 2 ng/mL). At a fentanyl concentration of 1.7 ng/mL, the MAC of isoflurane is reduced by 50%.21 Once the fentanyl concentration is increased beyond 3 ng/mL, there appears to be minimal further reduction with a maximum MAC reduction of 80%. Likewise, the MAC of desflurane is reduced by approximately 50% 25 minutes after a 3-μg/kg IV bolus of fentanyl.22 However, when the fentanyl bolus is increased to 6 μg/kg, there is no significant further decrease in the MAC of desflurane. Studies with other opioids have yielded similar results.23,24,25 The interactions between propofol and opioids are important because these agents are frequently used during monitored anesthesia care. When analgesic concentrations of fentanyl (0.6 ng/mL) are used in combination with propofol for anesthesia, the Cpss50 of propofol is reduced by 50% compared with when propofol is used as the sole agent.17 However, when the dose of fentanyl is increased, there is no significant further reduction of the Cpss50 for propofol beyond a fentanyl concentration of 3 ng/mL.
Opioid and benzodiazepine combinations are frequently used to achieve the components of hypnosis, amnesia, and analgesia. This drug combination displays marked synergism in producing hypnosis. Approximately 25% of the median effective dose for each individual drug is required in combination to induce hypnosis in 50% of patients.26 If the combination were simply additive, hypnosis would be induced in only approximately 25% of patients. Even subanalgesic doses of alfentanil (3 μg/kg) produce a profound reduction in midazolam requirements for hypnosis.27 This synergism also extends to the unwanted effects of these drugs, producing the life-threatening complications of respiratory and cardiac depression. Several fatalities have been reported after the use of midazolam, the majority of these being related to adverse respiratory events.28 In many of these cases, midazolam was used in combination with an opioid. The effects of midazolam and fentanyl on respiratory function in healthy volunteers have been examined by Bailey et al.29 Whereas midazolam produced no significant respiratory effects alone, and fentanyl alone produced hypoxemia (oxyhemoglobin saturation 95%) in half of the subjects, the combination of midazolam 0.05 μg/kg and fentanyl 2 μg/kg resulted in hypoxemia in 11 of 12 subjects and apnea (no spontaneous respiratory effort for 15 seconds) in 6 of 12 subjects. The combination of midazolam and fentanyl places patients at high risk for developing hypoxemia and apnea. The respiratory depressant effects of this drug combination are likely to be even more significant in the patient with coexisting respiratory or central nervous system disease or at the extremes of age. In clinical practice, the clinical advantages of the synergy between opioids and benzodiazepines for the maintenance of patient comfort should be carefully weighed against the disadvantages of the potentially adverse effect of this drug combination on the cardiovascular and respiratory systems.
Specific Drugs used for Monitored Anesthesia Care
Propofol
Over the past 30 years, propofol has become a popular choice for monitored anesthetic care due to its side effect profile and ease of titratability. Propofol has many of the ideal properties of a sedative–hypnotic for use in monitored anesthesia care. Its pharmacokinetic profile, that is, a context-sensitive half-time that remains short even after infusions of prolonged duration and a short effect–site equilibration time makes it an easily titratable drug with an excellent recovery profile. The quality of recovery and the low incidence of nausea and vomiting make propofol particularly well suited to ambulatory monitored anesthesia care procedures. A significant body of experience with the use of propofol for monitored anesthesia care has emerged. Propofol has significant advantages compared with benzodiazepines when used as the hypnotic component of a monitored anesthesia care technique. Although midazolam has a relatively short elimination half-time, its context-sensitive half-time is approximately twice that of propofol. Whereas propofol is noted for the rapid return to clear-headedness midazolam is often associated with prolonged postoperative sedation and psychomotor impairment, particularly in the elderly. Propofol in typical monitored anesthesia care doses (25 to 75 μg/kg/min) has minimal analgesic properties although propofol use during anesthesia has been associated with less postoperative pain and narcotic use when compared to isoflurane.30 However, the unique advantages of propofol can be exploited to the maximum when propofol is used to provide sedation when the analgesic component is provided by a local or regional analgesic technique. The use of propofol (50 to 70 μg/kg/min) to provide sedation (defined as sleep with preservation of the eyelash reflex and purposeful reaction to verbal or mild physical stimulation) as an adjunct to spinal anesthesia for lower limb surgery has been examined.31,32,33 After termination of infusions of approximately 100 minutes, patients regained consciousness in approximately 4 minutes. The authors also noted the ease with which general anesthesia could be induced if necessary by increasing the propofol infusion. The same group also compared propofol (60.5 μg/kg/min) with midazolam (4.3 μg/kg/min) as an adjunct to spinal anesthesia. The propofol group had faster immediate recovery than the midazolam group (2.3 vs. 9.2 minutes to spontaneous eye opening). Furthermore, psychomotor function was comparable with baseline values following propofol
sedation but did not return to baseline until 2 hours after midazolam administration. Several studies comparing propofol and midazolam sedation for local and regional anesthesia demonstrated that propofol produced less postoperative sedation, drowsiness, confusion, and clumsiness than midazolam but that discharge times varied.34,35
sedation but did not return to baseline until 2 hours after midazolam administration. Several studies comparing propofol and midazolam sedation for local and regional anesthesia demonstrated that propofol produced less postoperative sedation, drowsiness, confusion, and clumsiness than midazolam but that discharge times varied.34,35
Table 29-1. Published Strategies for Reducing the Pain on Intravenous Injection of Propofol | |
|---|---|
|
General anesthesia with propofol is generally associated with less nausea and vomiting than most other anesthetic techniques.36,37,38,39,40,41 There is growing evidence that even subhypnotic doses of propofol also possess direct antiemetic properties particularly when combined with an antiemetic in patients at risk for nausea and vomiting.42,43,44,45 Thus, it is likely that the beneficial effects of propofol upon nausea and vomiting will be a feature of monitored anesthesia care techniques using this drug. On the other hand, even during low-dose infusions used for sedation, pain during injection of propofol may be troublesome in 33% to 50% of patients.35,46 Several strategies for reducing the pain of propofol administration are described in Table 29-1.47
Fospropofol
In December 2008, fospropofol, a prodrug of propofol, was approved by the FDA for use during monitored anesthetic care, but at this time, it has not been extensively studied. This phosphate ester prodrug is metabolized by endothelial cell alkaline phosphatases to intermediate metabolites of propofol, formaldehyde, and phosphate.48,49,50 Recent publications on the pharmacodynamics and pharmacokinetics of fospropofol were retracted due to an error discovered in the analytical propofol assay.51,52,53 It is known that the active metabolite of propofol has a Cmax value of 4 minutes which is longer than the lipid-based fospropofol formulation. Information obtained from the manufacturer’s website listed the terminal phase elimination half-life (t1/2) of fospropofol as 0.81 ± 0.08 and 0.88 ± 0.08 hours in healthy subjects and patients, respectively. In healthy subjects, the apparent total body clearance of liberated propofol was 1.95 ± 0.345 L/hr/kg and t1/2 was 2.06 ± 0.77 hours. In patients, clearance of fospropofol was 0.31 ± 0.14 L/hr/kg, and clearance for propofol was 2.74 ± 0.80 L/hr/kg and is similar to that observed in healthy subjects. Pharmacokinetics of fospropofol does not appear to be affected by patients with mild to moderate renal insufficiency. At this time, it has not been studied in patients with hepatic impairment.
The standard dosing regimen recommended by the manufacturer Eisai is an initial IV bolus dose of 6.5 mg/kg followed by supplemental doses of 1.6 mg/kg as needed. No initial dose should exceed 16.5 mL; no supplemental bolus should exceed 4 mL. If patients are greater than 65 years of age or classified as ASA physical status 3 or 4, a modified dosing regimen (a 25% reduction of the standard dose), should be administered. Supplemental doses should only be administered after patients can demonstrate movement upon command (verbal or tactile stimulation) and not more frequently than every 4 minutes (accessed from Eisai website January 2012 URL http://us.eisai.com/product.asp?ID=274).
At the time of this writing, there are limited numbers of clinical control trials to evaluate the safety and efficacy of fospropofol for short procedures requiring monitored anesthesia care. Cohen et al.54 conducted a randomized controlled multicenter trial of 127 ASA PS range from 1 to 4 undergoing colonoscopy. The investigators randomized patients into two main treatment arms (fospropofol vs. midazolam). The fospropofol group was further randomized into one of four treatment arms of increasing doses of fospropofol (2 mg/kg, 5 mg/kg, 6.5 mg/kg, or 8 mg/kg). All patients were pretreated with 50 μg of fentanyl. They concluded that the optimal dose to provide moderate sedation was 6.5 mg/kg. The higher dose of 8 mg/kg met the criteria for deep sedation and was not necessary in order to complete the procedure. The most common adverse effects reported included paresthesias described as a burning sensation in the perineal and perianal area, pruritus, hypoxemia, hypotension, and abdominal pain. An additional follow-up publication by the same investigators analyzed 314 patients and concluded that the appropriate dose for moderate sedation was 6.5 mg/kg. At this dosage, they reported a 4% incidence of deep sedation.55
Fospropofol was also studied as the sedative agent for patients undergoing bronchoscopy. This study is a multicentered double blinded randomized controlled study of 252 patients classified as ASA P1 to P4 undergoing flexible bronchoscopy. All patients received 50 μg of fentanyl prior to the administration of either 2 mg/kg or 6.5 mg/kg fospropofol. The investigators reported a higher success rate with the higher dose of 6.5 mg/kg compared to 2 mg/kg (88.7 vs. 27.5% respectively; p < 0.001). The most common adverse events reported included paresthesias (47.6%), pruritus (14.7%), hypoxemia (14.3%), and hypotension (3.2%). Approximately one-third of patients who received the 6.5 mg/kg dose required some form of airway support. There was a higher satisfaction rate of both physician and patient when the 6.5 mg/kg dose was administered.
It should be noted that these studies were fully sponsored by the manufacturer and conducted by nonanesthesiologists. One of the investigators is a consultant for the manufacturer MGI/Eisai corporation. The manufacturer attempted to obtain FDA approval for nonanesthesiologists to provide fospropofol for monitored anesthesia care; however, this request was later denied. The Drug Enforcement Administration listed fospropofol as a class IV controlled substance.
Benzodiazepines
Benzodiazepines are commonly used during monitored anesthesia care for their anxiolytic, amnestic, and hypnotic properties. Patients presenting for diagnostic and surgical procedures frequently request some form of anxiolytic. Midazolam is usually administered prior to the start of the surgical or diagnostic procedures to facilitate amnesia and reduce the patient’s level of anxiety. Compared to other benzodiazepines, midazolam’s relatively
short elimination half-life and decreased likelihood of concomitant drug interactions makes this a superior choice to other benzodiazepines. The important differences between midazolam and diazepam are listed in Table 29-2.56 Although midazolam has a short elimination half-time, there is often significant and prolonged psychomotor impairment following sedation techniques using midazolam as a significant component. With the recent availability of propofol, midazolam may be better used in a modified role by using lower doses prior to the start of a propofol infusion to provide the specific amnestic and perhaps anxiolytic component of a “balanced” sedation technique rather than as the major hypnotic component.57 A study in healthy volunteers demonstrated that propofol reduced the distribution and clearance of midazolam in a concentration-dependent manner. The group reported increased plasma levels of midazolam ranging from 5% (±14.7%) to 26% (±9.4%) during increasing doses of propofol for monitored anesthesia care. This strategy allows the more evanescent and titratable propofol to provide the desired level of deep sedation in an adjustable manner according to the specific stimulus. The analgesic component, if required, of a balanced monitored anesthesia care technique may be provided by regional/local techniques or opioids. Again, when using opioids with benzodiazepines, the potential for significant respiratory impairment should be considered.
short elimination half-life and decreased likelihood of concomitant drug interactions makes this a superior choice to other benzodiazepines. The important differences between midazolam and diazepam are listed in Table 29-2.56 Although midazolam has a short elimination half-time, there is often significant and prolonged psychomotor impairment following sedation techniques using midazolam as a significant component. With the recent availability of propofol, midazolam may be better used in a modified role by using lower doses prior to the start of a propofol infusion to provide the specific amnestic and perhaps anxiolytic component of a “balanced” sedation technique rather than as the major hypnotic component.57 A study in healthy volunteers demonstrated that propofol reduced the distribution and clearance of midazolam in a concentration-dependent manner. The group reported increased plasma levels of midazolam ranging from 5% (±14.7%) to 26% (±9.4%) during increasing doses of propofol for monitored anesthesia care. This strategy allows the more evanescent and titratable propofol to provide the desired level of deep sedation in an adjustable manner according to the specific stimulus. The analgesic component, if required, of a balanced monitored anesthesia care technique may be provided by regional/local techniques or opioids. Again, when using opioids with benzodiazepines, the potential for significant respiratory impairment should be considered.
Table 29-2. Comparison of the Important Properties of Midazolam and Diazepam | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Age of the patient should be taken into consideration when administering benzodiazepines. The dose of a particular benzodiazepine required to reach a desired clinical end point is reduced in elderly compared with younger patients.58 This difference in dosing requirements in elderly patients is mainly related to pharmacodynamic factors. As demonstrated by the threefold decrease in plasma concentration of midazolam, 50% of patients would be expected not to respond to verbal command (Cp50) in an 80-year-old patient compared with a 40-year-old patient (Fig. 29-4).59
Benzodiazepines are valuable components of monitored anesthesia care techniques because they enhance patient comfort, improve operating conditions, and provide amnesia. However, recovery of psychomotor and cognitive function may be significantly prolonged following benzodiazepine sedation, especially when compared with sedative–hypnotic techniques using propofol as the major component.60 The specific benzodiazepine antagonist flumazenil provides the potential to improve the recovery profile of benzodiazepines by permitting the active termination of their sedative and amnestic effects without invoking adverse side effects. However, the potential for resedation remains an obstacle to the routine use of benzodiazepine reversal, particularly in patients undergoing ambulatory procedures. The effects of midazolam may recur up to 90 minutes following the administration of flumazenil.46 Thus it is possible that patients could be discharged prematurely to a less well-monitored area, or even out of the hospital in the case of ambulatory surgery, and later experience recurrence of benzodiazepine effects. An important additional issue is that of cost. The routine use of flumazenil-antagonized benzodiazepine sedation has a significant cost disadvantage. Ghouri et al.46 demonstrated that flumazenil-antagonized midazolam sedation was more expensive than propofol sedation ($68.67 vs. $27.80). Typical dose requirements for use of flumazenil are listed in Table 29-3.
Opioids
Opioids are administered in the context of monitored anesthesia care to provide the specific analgesic component of a “balanced” technique. Opioids are indicated when regional or local anesthetic techniques are inappropriate or ineffective and are typically administered immediately prior to the painful or invasive
portion of the procedure. In addition, opioids may be indicated to blunt untoward hemodynamic and physiologic responses, a desirable effect in patients with significant cardiac disease. Pain relief may be required for factors other than the procedure itself, such as uncomfortable positioning, propofol injection, pneumatic tourniquet pain, or other pain not relieved by the local anesthetic technique. The choice of a particular opioid depends on several factors including cost, availability, time of onset, duration, and potential side effects. Opioids frequently administered during monitored anesthesia care include alfentanil, fentanyl, and remifentanil. Their adverse effects include respiratory depression, muscle rigidity, and nausea and vomiting, all of which are undesirable in the spontaneously breathing patient with an unprotected airway. A complicating issue is that the ability to predict the effect of a given dose of opioid in a particular patient is limited by significant interpatient pharmacokinetic and pharmacodynamic variability. Furthermore, the coadministration of sedative agents increases the risk of serious adverse events, particularly respiratory arrest. This problem is usually overcome in practice by the cautious incremental administration of small, carefully titrated boluses or by titrating infusions to the desired effect.
portion of the procedure. In addition, opioids may be indicated to blunt untoward hemodynamic and physiologic responses, a desirable effect in patients with significant cardiac disease. Pain relief may be required for factors other than the procedure itself, such as uncomfortable positioning, propofol injection, pneumatic tourniquet pain, or other pain not relieved by the local anesthetic technique. The choice of a particular opioid depends on several factors including cost, availability, time of onset, duration, and potential side effects. Opioids frequently administered during monitored anesthesia care include alfentanil, fentanyl, and remifentanil. Their adverse effects include respiratory depression, muscle rigidity, and nausea and vomiting, all of which are undesirable in the spontaneously breathing patient with an unprotected airway. A complicating issue is that the ability to predict the effect of a given dose of opioid in a particular patient is limited by significant interpatient pharmacokinetic and pharmacodynamic variability. Furthermore, the coadministration of sedative agents increases the risk of serious adverse events, particularly respiratory arrest. This problem is usually overcome in practice by the cautious incremental administration of small, carefully titrated boluses or by titrating infusions to the desired effect.
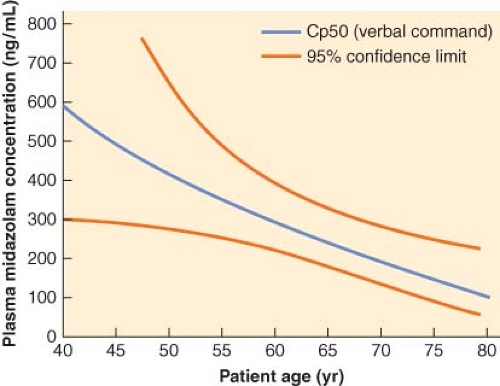 Figure 29.4. Midazolam Cp50 (the concentration at which 50% of subjects will fail to respond to a verbal command) as a function of age. There is a marked decrease in midazolam requirements as patient age increases. (Reproduced from Jacobs JR, Reves JG, Marty J, et al. Aging increases pharmacodynamic sensitivity to the hypnotic effects of midazolam. Anesth Analg 1995;80:143, with permission.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|