Fig. 48.1
CT scan of the chest; arrow points out the catheter in first image; second image shows bright-smeared impression of the catheter abutting the parietal pleura; patient with pleuritic pain from irritation of pleura by the migrated catheter. The arrows point toward the catheter and its proximity to the pleura. Image from personal library
However, during the morning hours, another attempt was made to remove the catheter under fluoroscopic guidance. In the pain clinic, the catheter was clearly visible under fluoroscopic guidance. The area around the catheter was cleaned with an alcohol-based chlorhexidine solution and draped. Lidocaine 1% was injected to numb the area around the catheter. The injection was done under fluoroscopy guidance to ensure that the needle did not contact the catheter to avoid catheter shearing or breakage. A 16 g angiocath cannula was threaded over the catheter, under fluoroscopy guidance, up to the hub, in order to mobilize the tissue around it, ensuring again that catheter did not break under the skin. Under fluoroscopic guidance, we verified that that catheter did not kink or that a piece of it did not break off during tugging or mobilization maneuvers (Figs. 48.2 and 48.3).
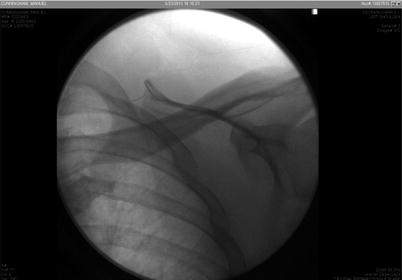
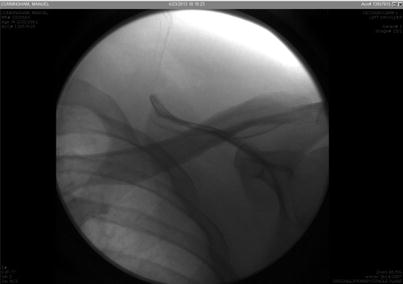

Fig. 48.2
Catheter as seen under fluoroscopy when removing efforts started. Image from personal library

Fig. 48.3
Fluoroscopic image after catheter has been removed. There is no piece left behind in the patient’s body; all pieces were removed. Image from personal library
After repeated gentle tugging, the catheter was withdrawn with the tip intact. The thoracotomy was canceled, and the patient was discharged home from the pain clinic (Figs. 48.4 and 48.5).
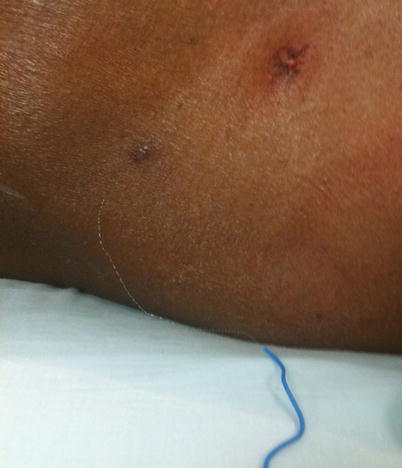
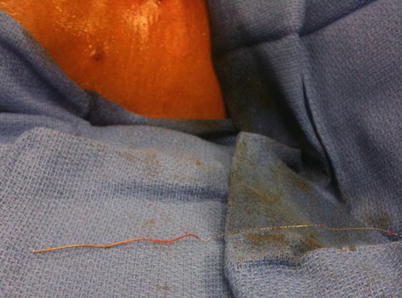

Fig. 48.4
Sheered catheter from attempt to remove it in the ER. Image from personal library

Fig. 48.5
Catheter removed from patient body with tip intact after procedure performed under direct fluoroscopy. Image from personal library
48.2 Case Discussion
Complex regional pain syndrome (CRPS) is a chronic painful condition. Its prevalence is reportedly around <2% in most retrospective series [1]. A study from the Netherlands reported an incidence of 26.2 cases per 100,000 person-years; a study from the United States estimated the incidence at 5.5 cases per 100,000 person-years [2, 3].
CRPS is a complicated condition to manage. No abnormal diagnostic test proves or disproves the diagnosis. Currently the diagnosis is made from the Budapest criteria, which rely on the presence of symptoms and signs in four different categories [4] (Table 48.1).
Table 48.1
Clinical diagnostic criteria for complex regional pain syndrome—Budapest criteria
1. Continuing pain disproportionate to the inciting event |
2. At least one symptom in one of three of the four following categories Sensory Motor/trophic Sudomotor/edema Vasomotor |
3. At least one sign at the time of evaluation in two or more of the following categories Sensory Motor/trophic Sudomotor/edema Vasomotor |
4. No other diagnosis explains the signs and symptoms |
CRPS I = no major nerve damage; CRPS II = major nerve damage |
In the absence of any other explanation and the presence of any symptoms in three out of four categories and any sign in two of the four other categories, the diagnosis is made [4] (Table 48.2).
Table 48.2
Common clinical characteristics of complex regional pain syndrome
Diagnostic categories | Symptoms | Signs |
|---|---|---|
Sensory | Hyperesthesia and/or allodynia | Hyperalgesia (to pinprick) and/or allodynia (to light touch and/or temperature sensation and/or deep somatic pressure and/or joint movement) |
Motor/trophic | Decreased range of motion and/or motor dysfunction (weakness, tremors, dystonia) and/or trophic changes (hair, nail, skin) | Decreased range of motion and/or motor dysfunction (weakness, tremor, dystonia) and/or trophic changes (hair, nail, skin) |
Vasomotor | Temperature asymmetry and/or color changes and/or color asymmetry
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|



