189 Methamphetamine, Ecstasy, and Other Street Drugs
 History
History
The first documented synthesis of methamphetamine, utilizing ephedrine as a substrate, occurred in 1918 in Japan. A closely related compound, d-amphetamine, had been created 20 years earlier in Germany (Figure 189-1). These compounds are not found naturally, although similar substances such as ephedra (ma-huang), cathine, and cathinone have stimulant properties and are extracted from certain plants. Both amphetamine and methamphetamine originally were used in nasal decongestants and bronchial inhalers beginning in the 1930s. A research report in 1937 claimed amphetamine enhanced work output and intellectual performance, and the legal use of amphetamine increased as a result.1 During this period, amphetamines were noted to reduce impulsive behavior and hyperactivity in children.2 In 1954, methylphenidate (Ritalin, Concerta, Methylin) was approved for this indication. There are a variety of these drugs with slight differences in chemical structure. For example, Adderall combines dextroamphetamine and amphetamine with d-amphetamine saccharate and d,l-amphetamine aspartate. In the fenethylline (Captagon) molecule, theophylline is covalently linked with amphetamine via an alkyl chain.
Millions of doses of amphetamine and methamphetamine were taken by military personnel of all nations during World War II.3 Thereafter, surplus supplies entered civilian markets, most notably Japan. Methamphetamine was widely prescribed for depression and obesity, reaching a peak of 31 million prescriptions in the United States in 1967. During this period, production and distribution was largely controlled by motorcycle gangs who hid the drugs in the crankcase of their motorcycles (hence the street name, “crank”). Amphetamines were outlawed after the United States Drug Abuse and Regulation Control Act of 1970. In the 1980s, a concentrated form of methamphetamine, known as “ice,” “glass,” or “crystal,” became popular.4 Production and trafficking in the United States continues to increase, with significant input from Mexican, Southeast Asian, West African, and European drug cartels. An estimated 12.3 million Americans have tried methamphetamine at least once, and 600,000 are weekly users.5 The global number of users is estimated to be over 50 million. Methamphetamine is the most prevalent illegally manufactured controlled substance in the United States and worldwide.6 Abuse of legally prescribed amphetamines represents a new problem among teenagers and young adults.7
 Pharmacology and Metabolism
Pharmacology and Metabolism
Methamphetamine can be ingested orally, snorted, injected, or smoked. It is lipid soluble and crosses the blood-brain barrier readily. Methamphetamine acts primarily on dopaminergic central nervous system (CNS) cells. In small doses, amphetamines cause release of dopamine from the cytoplasmic pool by exchange diffusion at the membrane dopamine uptake transporter locus.8 Methamphetamine also antagonizes the reuptake of catecholamines by competitive inhibition. As the dose increases, amphetamines diffuse through the presynaptic terminal membrane and bind to the neurotransmitter transporter on the vesicular membrane, resulting in the exchange release of dopamine into the cytoplasm (Figure 189-2). Dopamine is then released into the synapse by reverse transport at the dopamine uptake locus. At even higher doses, methamphetamine diffuses through the cellular and vesicular membranes and alkalinizes the vesicles. This results in dopamine release from the vesicles and delivery into the synapse by reverse transport. Chronic methamphetamine use results in down-regulation of dopamine D1 and D2 receptors.9
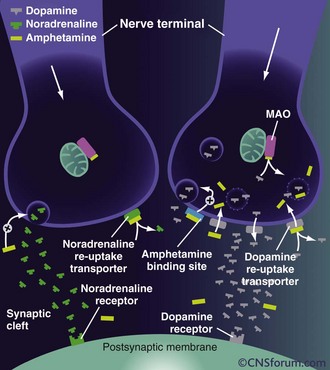
Figure 189-2 Mechanisms of action of high-dose amphetamine on central dopamine and norepinephrine transmission.
(From www.cnsforum.com, with permission.)
Increased norepinephrine at the locus ceruleus results in anorectic and locomotor effects. Increased dopamine in the neostriatum results in glutamate release and inhibition of γ-aminobutyric acid (GABA)-ergic neurons.10 There is evidence that glutamate stimulation contributes significantly to the neurotoxicity of amphetamines.11 The serotonin transporter exhibits abnormal efflux in response to amphetamines.12 Elevated serotonin and dopamine levels within the mesolimbic system may cause hallucinations and psychosis. Release of dopamine in the ventral tegmental area is involved in reward and addiction.13 The rage reaction induced by amphetamines may result from increased release of dopamine in the limbic system.14
Metabolism of methamphetamine occurs in the liver. Dealkylation and demethylation are performed by cytochrome P450 isoenzymes. Metabolites include amphetamine, 4-hydroxymethamphetamine, and 4-hydroxyamphetamine, which are also biologically active stimulants.15 The pharmacokinetics are complex and nonlinear.16 The biological half-life varies from 6 to 15 hours after a single dose. However, the cellular effects may last for days. Excretion occurs primarily in the urine and is affected by pH. Amphetamines are basic, with a typical pKa range from 9 to 10, and renal elimination is enhanced with acidic urine. Approximately 60% of an oral dose is eliminated in the urine within the first 24 hours, with about one-third as intact drug and the remainder as metabolites.
 Acute Effects
Acute Effects
Methamphetamine abusers may present acutely with myriad symptoms including agitation, confusion, tremors, anxiety, hyperthermia, hypertension, tachycardia, and seizures. Less common effects include arrhythmias, cerebral and pulmonary edema, hepatotoxicity, and disseminated intravascular coagulation. Patients may present with atypical chest pain and be at risk for acute coronary syndrome.17 Compared to cocaine, methamphetamine is less likely to cause myocardial ischemia, as it does not interfere with thromboxane production and platelet aggregation.18 Severe abdominal pain may be the result of acute mesenteric vasoconstriction. Necrotizing vasculitis is associated with methamphetamine abuse and can involve multiple organ systems.19 Methamphetamine patients utilize prehospital, emergency department, and hospital resources at a much higher than average rate.20 Methamphetamine crosses the placenta, and use during pregnancy may result in fetal growth retardation, premature birth, developmental delay in neonates, and cognitive deficits in children.21
A dangerous stage of methamphetamine toxicity occurs when an abuser has not slept for days and is irritable and paranoid. This behavior is referred to as “tweaking.” The individual craves more methamphetamine, but it is impossible to achieve the original high, causing unpredictable, unstable, and violent behavior.22 Abusers frequently neglect nutrition and fluid intake during these periods, which can result in rhabdomyolysis when combined with periods of agitation or long periods of inactivity (Figure 189-3).23 The proliferation of clandestine methamphetamine laboratories has increased the incidence of chemical and thermal burn injuries.24 Lead and mercury contamination from illicit production of methamphetamine may result in acute toxicity.25
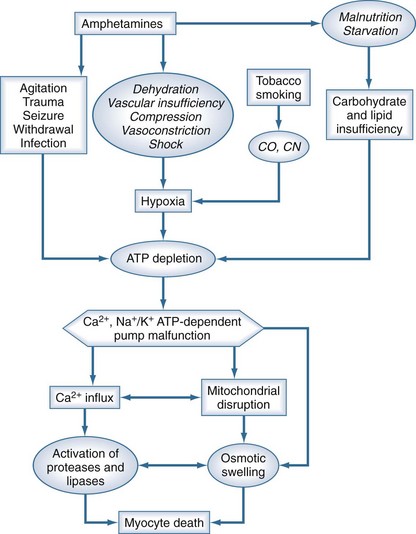
Figure 189-3 Putative relationship between amphetamines and other cofactors with development of rhabdomyolysis.
Chronic use of methamphetamine results in numerous harmful and irreversible cellular and end-organ effects. Long-term use has been shown to result in unique patterns of periodontal disease and tooth loss, a condition commonly referred to as “meth mouth” (Figure 189-4); the maxillary teeth tend to be most prominently affected.26 Chronic users also develop characteristic skin lesions such as prurigo nodularis, also known as “speed bumps,” from constant picking and scratching, usually from delusions of parasitosis. Methamphetamine is also neurotoxic to dopaminergic and serotoninergic cells.27 Chronic users may develop a syndrome similar to Parkinson’s disease.28 Unusual choreoathetoid movements result from increased dopaminergic activity within the striatal area.29 A magnetic resonance imaging (MRI) study of methamphetamine addicts demonstrated permanent gray-matter deficits of the cingulate, limbic, and paralimbic cortices and white matter hypertrophy, which correlated with memory and mood disorders exhibited by the subjects.30 Chronic methamphetamine use results in inhibition of tyrosine hydroxylase, deficits in mitochondrial energy production, and neuronal apoptosis from oxidative stress.31
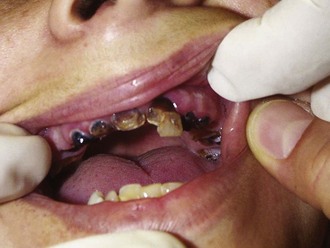
Figure 189-4 Anterior maxillary tooth loss associated with chronic methamphetamine abuse (ungloved finger is the patient’s).
Chronic use has been demonstrated to result in cardiomyopathy, congestive heart failure, and accelerated coronary artery disease.32 Methamphetamine use often results in impulsive behavior, which facilitates transmission of sexually transmitted diseases, viral hepatitis, and human immunodeficiency virus (HIV).33 Furthermore, for those injecting the drug, bacterial and foreign-body contamination may result in endocarditis, tetanus, wound botulism, osteomyelitis, and pulmonary and soft-tissue abscesses.34 Methamphetamine withdrawal syndrome is initially characterized by increased sleep, eating, and dysphoria. This pattern declines into a subacute phase lasting up to 2 weeks.35 These symptoms, coupled with increased risk-taking behavior, result in a higher incidence of traumatic injuries from motor vehicle accidents, falls, and assaults.36 Methamphetamine users often exhibit suspicion and paranoia and are rarely forthcoming about the details of their drug use.37 This pattern includes denial even when presented with a positive toxicology screen or other physical evidence.
 Clinical Management
Clinical Management
Acute methamphetamine toxicity is a true emergency. Airway, breathing, circulation, and temperature should be assessed, with continuous telemetry monitoring and frequent measurement of blood pressure. For patients with hyperthermia, a rectal thermometer is the most accurate method to trend core temperature. Agitated patients initially should be restrained physically, using a team approach (one person per limb) to protect both the patient and staff from harm. This form of restraint should immediately be followed by chemical restraint, using neuroleptic agents or benzodiazepines. Once adequate sedation has been achieved, physical restraints should be removed as soon as feasible. Neuroleptic agents may have a theoretical advantage over benzodiazepines, as these are CNS dopamine antagonists and may directly counteract the excess levels of dopamine in the CNS which result from methamphetamine abuse.37–41 Furthermore, neuroleptics do not affect respiratory drive, whereas multiple or large doses of benzodiazepines may result in respiratory depression. For benzodiazepines, typical dosages are 5 to 10 mg of diazepam or 1 to 4 mg of lorazepam by intravenous (IV) route.
The authors of a prospective study that compared lorazepam with droperidol for control of agitated methamphetamine users concluded that droperidol was longer acting than lorazepam and required fewer repeat doses to achieve sedation.37 Droperidol, or the longer-acting agent, haloperidol, may be administered by either the IV or intramuscular route. Typical doses required are 2.5 to 5 mg of droperidol or 5 to 10 mg of haloperidol. Of note, use of the butyrophenones for this particular indication is considered “off-label.” Droperidol, however, has been used safely for over 3 decades as an antiemetic, and millions of units have been dispensed; nevertheless, concern for QT-interval prolongation and development of torsades de pointes has resulted in a controversial U.S. Food and Drug Administration (FDA) Black Box warning for doses above 2.5 mg.42 Haloperidol is not FDA approved for IV use and also has a Black Box warning for use in elderly patients with dementia. Newer antipsychotics such as olanzapine and ziprasidone also may be effective, but they are not FDA approved for IV use and have not been studied in depth for this indication.43–45 For patients who do not respond to these pharmacologic interventions, rapid-sequence induction using paralytic agents and endotracheal intubation and mechanical ventilation may be required.