Evidence-Based Medicine
The formal definition of evidence-based medicine (EBM) is “the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients.”1 This definition is useful but leaves ambiguities. What does “explicit” mean? How might we define “judicious”? What constitutes “evidence”? And perhaps most important, who is “making decisions,” the doctor or the patient?
Broadly introduced to medical culture in 1992, EBM has exploded in the past 2 decades.2 The term has become ubiquitous and is often co-opted by working groups, professional societies, and individual authors who neither systematically adhere to core EBM principles nor complete the fundamental tasks that define EBM. In addition, the most important principle underlying medical practice and the Hippocratic Oath—that the physician’s goals should be aligned with the patient’s goals—has not been routinely integrated into these applications of EBM.
Clinical Scenarios
Acute Headache in the Emergency Department
Ask
The prevalence of SAH varies considerably across practice settings and research cohorts, which is often a reflection of biases such as spectrum bias, or enrollment of an overly narrow or broad spectrum of patients, and referral bias, which is one type of spectrum bias and typically occurs when a study enrolls only a referred patient cohort. A recent publication of patients referred to a neurosurgical department for headache suspicious of SAH found a remarkable prevalence of 59%.3 In contrast, in a large retrospective study of nearly all patients with headache encountered in an ED, only 1% were found to have SAH.4 In the second example, a broad cohort with limited inclusion or exclusion criteria related to headache quality was evaluated, and very few had the disease of interest. Conversely, when only a highly selected population of patients with headache referred to a neurosurgeon were studied, the majority had significant pathology. Ideally, any study that we use to generate a risk estimate for Ms. Mason should be from the ED setting and should enroll those with a similarly “acute” headache.
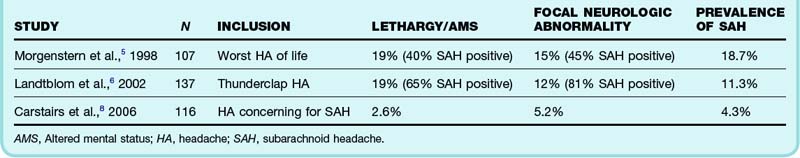
Further refining our question, we note that Ms. Mason is awake and alert with normal findings on neurologic examination. One review has suggested that 15% of patients seen in the ED with a thunderclap headache have SAH, but the two studies in this review did not distinguish between neurologically compromised and normal patients.5,6 In both studies, one in five patients enrolled was noted to have either lethargy or altered mental status. Not surprisingly, another prospective study in which fewer patients with abnormal neurologic findings were enrolled found a much lower prevalence of disease7 (Table 1).
Table 1 Prospective Studies Including Patients with Severe, Acute Headaches, Including Patients with Abnormal Findings on Neurologic Examination
Acquire
One of the first papers found is a recent publication by Perry et al.: “High Risk Clinical Characteristics for Subarachnoid Haemorrhage in Patients with Acute Headache: Prospective Cohort Study.”9 Scanning the abstract, we decide that it meets many of our important inclusion criteria and thus embark on the third part of the EBM process.
Apply
When the diagnosis of SAH is being pursued, the current recommended approach includes a CT scan, followed by LP when imaging is negative.10 Based on study design and disease prevalence, the sensitivity of CT has varied widely across research populations, between 82% and 100%, and this has been judged inadequate in the context of the disease.8 Although a recent investigation of CT for SAH reported a sensitivity approaching 100%, spectrum bias was evident because all the subjects were referred to a neurosurgical center for evaluation.3
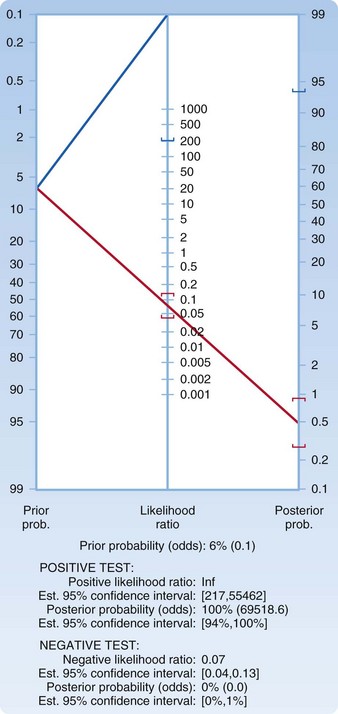
We decide to presume a worst-case scenario for the imaging test and choose an LR of 0.13, which represents the upper boundary of the confidence interval. Plotting a pretest probability of 6.5% on a Fagan nomogram and using an LR of 0.13, we generate a posttest probability of just under 1% (Fig. 1). You explain to the patient that a less than 1 in 100 chance (1 in 118 to be exact) persists that she has SAH detectable by LP. She carefully considers these numbers and asks another question: “How much lower would her risk be if LP were performed and determined to be normal?” In the EBM style of practice, one question often begets another. We return to the acquire and appraise steps.
The aforementioned literature search yields a paper by Perry et al.: “Is the Combination of Negative Computed Tomography and Negative Lumbar Puncture Result Sufficient to Rule Out Subarachnoid Hemorrhage?”11
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree