194 Management of the Postoperative Cardiac Surgical Patient
 The Cardiac Surgery Patient in the Intensive Care Unit
The Cardiac Surgery Patient in the Intensive Care Unit
History of Cardiac Surgery Linked to the History of Intensive Care
The development of modern cardiac surgery has been intimately related to the development of the ICU. This relationship has worked in both directions. Until the 1950s, cardiac surgery was limited to control of traumatic injuries and the closed repair of valves. Development of the extracorporeal pump oxygenator in 1953 by Gibbon ushered in the era of open-heart surgery.1 Heart valve replacement then became possible. Subsequently, in the 1960s, coronary artery bypass grafting (CABG) for ischemic heart disease was developed and rapidly popularized.2
Several studies have demonstrated that risk-adjusted mortality rates after CABG vary significantly among surgeons and hospitals and that mortality is related both to the number of surgeries performed by each surgeon and the total volume of procedures performed at the hospital.3–5 For high-risk surgical patients, survival is also related to the characteristics of the ICU care.6
The Changing Epidemiology of Cardiac Surgery
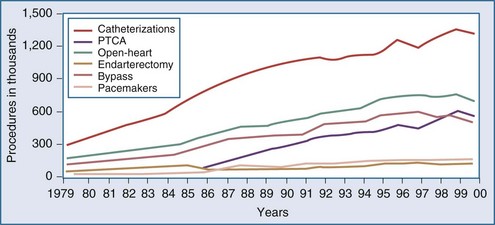
Over the last decade, the population of patients treated with cardiac surgery has changed dramatically. Advances in cardiology including reperfusion therapy, angioplasty, stenting, and drug-eluting stents, have obviated the need for surgical approaches to treatment except for particularly complex problems or after failure of other less invasive modalities. In the year 2000, 561,000 patients in the United States underwent percutaneous transluminal coronary angioplasty (PTCA), an increase of 262% relative to 1987. In the same year, 314,000 patients underwent CABG. Multiyear trends, represented in Figure 194-1, show a leveling off and subsequent decrease in the overall number of patients undergoing CABG.7 The recently developed sirolimus-coated coronary stent has been associated with even better results.8 Studies comparing the use of stents versus CABG for left main disease have found no significant difference in rates of death or of the composite endpoint of death, Q-wave infarction, or stroke between patients receiving stents and those undergoing CABG. However, stenting, even with drug-eluting stents, was associated with higher rates of target-vessel revascularization than was CABG.9
Alternative Techniques for Cardiac Surgery
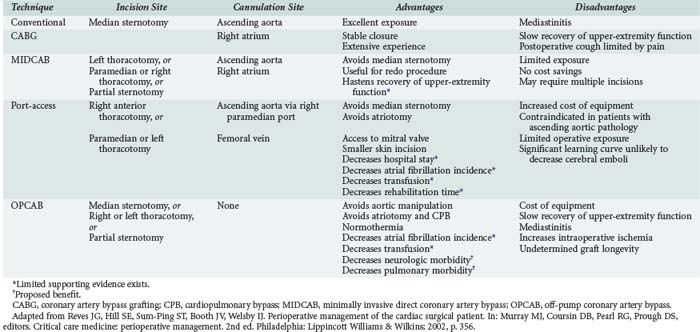
The increasing age of patients undergoing cardiac surgery and the relatively high incidence of adverse effects related to cardiopulmonary bypass (CPB) in these patients have led to the development of less invasive cardiac surgical techniques. These techniques are intended to decrease postoperative morbidity, reduce hospital length of stay, reduce costs, and hasten recovery of lifestyle (Table 194-1). Three major techniques have been proposed.
Minimally invasive direct coronary artery bypass (MIDCAB) differs from conventional CABG mainly in the type of incision used for access. In place of the conventional median sternotomy, access is obtained via a left or right thoracotomy, a parasternal incision, or a partial sternotomy. The proposed benefit of such an approach is the reduction in morbidity related to median sternotomy. This proposed advantage has not been demonstrated. MIDCAB grafting is a challenging technique and should be performed only in selected patients with favorable coronary anatomy. Both bare metal and drug-eluting stenting have been shown to be inferior to MIDCAB for proximal left anterior descending (LAD) coronary artery lesions, owing to higher reintervention rates with similar results in mortality and morbidity.4,10
Off-pump coronary artery bypass (OPCAB) is performed on a beating heart without benefit of CPB. The proposed benefit of this procedure is reduction of morbidity related to hypothermia and CPB. The procedure is undertaken using partial to full heparinization. Extubation may be achieved earlier in these patients because they do not require rewarming and are less coagulopathic. A subset of patients cannot tolerate the extent of retraction of the heart required for the surgery and need to be urgently placed on CPB. These patients may suffer ischemic myocardial injury and require support with inotropes or intraaortic balloon pumping (IABP) during the postoperative period. A retrospective study of 1398 patients showed that use of the OPCAB technique for multivessel myocardial revascularization in high-risk patients significantly reduced the incidence of perioperative myocardial infarction (MI) and other major complications, length of stay in the ICU, and mortality.11 In a single-center non-randomized registry, the incidence of major cardiac events were similar in OPCAB versus sirolimus-eluting stents in diabetic patients with multivessel disease.12
A third method of minimally invasive cardiac surgery is the port-access technique. This operation entails obtaining access for CPB with the use of endovascular catheters. This allows surgery to be performed using CPB via either a left or right thoracotomy. The technique is particularly useful for mitral valve replacement through a right thoracotomy and for redo CABG (avoiding the complications associated with repeat sternotomy). The port-access technique has been shown to be safe and is associated with shorter lengths of stay, reduced transfusion requirements, fewer infections, decreased incidence of renal failure, and less atrial fibrillation when compared with conventional techniques.13 In outcome data using propensity score analysis for mitral valve repair, minimally invasive repair had similar results to open repair. There was an increase in cross-clamp and bypass times, but early outcome was similar.14 Widespread adoption of this technique has been limited by the technical complexity of placing the required catheters, which requires both extra time and a specially trained and skilled operative team.
Organization of the Postoperative Cardiac Surgery Unit
Guidelines developed by the American Heart Association and the American College of Cardiology outline the requirements for cardiac surgical ICUs.15 These include the development of protocol-driven care, a minimum number of cardiac surgical ICU beds that is half the number of surgeries performed per week, and one-to-one nursing care during the first night in the unit. ICU coverage by a dedicated intensivist has been shown to improve outcomes in other types of major surgery and should be recommended after cardiac surgery as well.6
 Separation from Cardiopulmonary Bypass and the End of Surgery
Separation from Cardiopulmonary Bypass and the End of Surgery
Cardiopulmonary Bypass
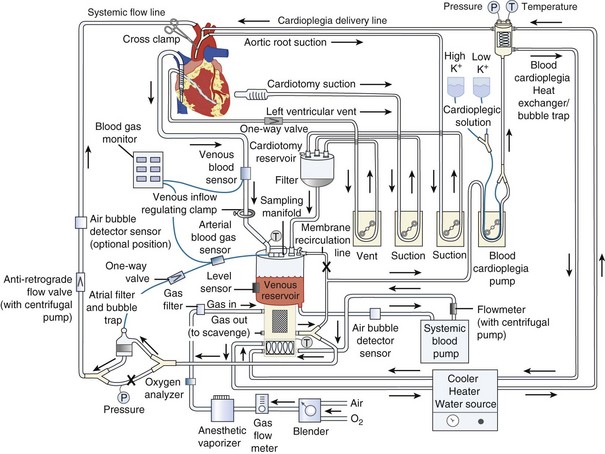
The goal of CPB is to separate the heart and lungs from the systemic circulation so that the heart can be arrested while the surgical repair is constructed. Blood is drained from the right side of the heart, either by gravity or with vacuum assistance, via a cannula in the right atrium directly or via a cannula in the femoral vein that is advanced into the right atrium. The blood is collected in a reservoir and then pumped through an oxygenator that contains a membrane where the blood is oxygenated and carbon dioxide is removed (Figure 194-2). The perfusionist controls both the fraction of inspired oxygen and the rate of oxygen flow through the circuit, thereby controlling the patient’s arterial oxygen and carbon dioxide levels, respectively. The treated blood then passes through an air filter and is returned to the patient via an arterial cannula placed in either the ascending aorta or the femoral artery. The perfusionist controls the amount of flow provided to the patient (i.e., CO). Mild to moderate systemic hypothermia (28°C-34°C) is used during bypass to minimize oxygen consumption by both the body and the brain. After adequate CPB is established, an aortic cross-clamp is applied to the ascending aorta, between the aortic cannula and the heart. The interval when the cross-clamp is applied is referred to as “ischemic” time, because no blood is circulated through the heart during this period. The heart is arrested by infusion of a high-concentration potassium solution into the native coronary arteries (antegrade cardioplegia) via a cannula placed between the aortic cross-clamp and the heart. Cardioplegia may also be given “backwards,” through the venous system of the myocardium (retrograde cardioplegia) via a catheter placed in the coronary sinus. Potassium is used as the arresting agent because it stops the heart from beating and minimizes myocardial oxygen consumption.
 Monitoring the Postoperative Cardiac Surgery Patient
Monitoring the Postoperative Cardiac Surgery Patient
Hemodynamic Monitoring
All patients admitted to the ICU after cardiac surgery will have their blood pressure continuously monitored using an intraarterial line. This is usually placed in either a radial or femoral artery. Accuracy of the measurements depends on strict attention to calibration, leveling, and removal of air from the tubing. After CPB, femoral arterial pressure may more accurately reflect central aortic pressures,16 but this problem has usually resolved by the time the patient arrives in the ICU. If the radial artery is cannulated, the hand should be examined for signs of ischemia.17 Vascular complications of femoral arterial lines are extremely rare, but femoral catheters may be associated with an increased incidence of infection.18
Central venous access is required in all patients for drug administration and hemodynamic monitoring. In the low-risk patient, a CVP catheter may be all that is needed, particularly if echocardiography is available as a backup. Pulmonary artery catheters have the advantage of allowing measurement of pulmonary artery occlusion pressure (PAOP), thermodilution, and CO, as well as sampling of the mixed venous blood saturation (SvO2). Use of the pulmonary artery catheter remains controversial. Improved outcome due to use of a pulmonary artery catheter for monitoring of cardiac surgical patients has not been demonstrated.19 Some studies showed an increased risk of death or adverse outcome when treatment was guided by the use of a pulmonary artery catheter.20,21 However, many of these studies have been criticized on methodological grounds, and use of the catheter in cardiac surgery remains widespread.22 Current guidelines recommend use of the pulmonary artery catheter in high-risk patients undergoing surgery in an appropriate practice setting.23 Such a setting is one in which the physician and nursing staff are familiar with the catheter and trained to properly interpret the information obtained. If echocardiography is readily available, it is possible to manage even high-risk patients using a CVP catheter.
Electrocardiography
Continuous ECG monitoring allows detection of arrhythmias. If an arrhythmia is detected, a 12-lead ECG should be obtained, and serum electrolyte concentrations should be measured. Treatment of arrhythmias should be carried out using established protocols.24 If a malignant arrhythmia occurs, myocardial ischemia should be considered as a possible precipitating cause.
Monitoring of trends in ST-segment elevation or depression allows early detection of postoperative myocardial ischemia. Although transient ST-segment changes are relatively common and of unclear significance, persistent changes should be investigated by obtaining a 12-lead ECG and measuring circulating levels of creatine kinase myocardial band (CK-MB), troponin-T, or troponin-I.25,26 If ischemia is strongly suspected, then echocardiography followed by coronary angiography should be considered. Findings from these studies may indicate the need for further coronary revascularization.
Echocardiography in the Intensive Care Unit
TEE is being used as a tool to facilitate decision making in the management of critically ill patients, including cardiac surgical patients. In the cardiac surgical ICU, this modality may have a particularly high yield when it is used to establish the cause of postoperative hypotension.27 In one large series, a new diagnosis was established or an important pathology was excluded in 45% of TEE examinations performed in the ICU. Pericardial tamponade was diagnosed in 34 cases (11%) and excluded in 36 cases (12%). Other diagnoses included severe left ventricular failure and presence of large pleural effusions. The results of TEE had an impact on therapy in 220 cases (73%) by leading to a change of pharmacologic treatment and/or fluid administration, reoperation, or a decision that reoperation was unnecessary.28
 Clinical Manifestations of the Postbypass Period
Clinical Manifestations of the Postbypass Period
The Normal Course
Patients are typically admitted to the ICU intubated and ventilated. Sedation with a short-acting agent, typically propofol, is continued until the patient is ready for extubation. Once hemodynamic stability is ascertained and chest tube drainage is judged to be under control, the patient is allowed to awaken. There is no need for prolonged weaning from mechanical ventilation. A short trial of spontaneous ventilation is sufficient to determine whether respiration will be adequate without mechanical support. The rapid shallow breathing index (RSBI) has been shown to be a sensitive way to assess the likelihood of successful extubation.29 The RSBI is calculated by dividing the respiratory rate (in breaths per minute) by the tidal volume (in liters). A value of lower than 105 predicts successful extubation. Chest tubes are commonly removed on the first postoperative day. The pulmonary artery catheter, if present, is discontinued, and the patient may be transferred to a step-down unit.
Fast-tracking of cardiac surgical patients refers to a comprehensive program designed to reduce both length of stay and hospital costs.30,31 As a part of this program, multiple anesthetic techniques designed to allow earlier postoperative extubation have been proposed, studied, and shown to be safe. These techniques may allow extubation in the OR.32 The key to proper use of this technique is patient selection. Although the criteria are expanding, patients with unstable angina or a high degree of congestive heart failure are generally not appropriate candidates for fast-tracking. In a retrospective review comparing 4020 patients undergoing cardiac surgery with a conventional anesthetic versus 3969 patients with a fast-track anesthetic, the fast-track group had shorter extubation times, shorter ICU or PACU stays, and a lower incidence of low cardiac output syndrome.
Low Cardiac Output
Preload
In some cases, low preload is not caused by absolute hypovolemia but by relative or distributional hypovolemia. CPB and subsequent rewarming may lead to vasodilatation and a subsequent hypotension. Intravascular volume expansion may be required to maintain perfusion. An acceptable alternative is administration of a low dose of vasopressor such as phenylephrine or norepinephrine to maintain an adequate perfusion pressure. Recently, vasopressin in doses between 0.01 and 0.1 units/min has been demonstrated to be effective in this situation.33,34 Vasodilatation is usually a transient problem that resolves during the first several hours after separation from CPB. Continued vasodilatation after this period should prompt a search for another cause, particularly infection.
Pump Failure
Either or both ventricles may fail postoperatively. Decreased myocardial contractility may be caused by impaired preoperative function, inadequate revascularization at surgery, post-CPB reperfusion injury, or perioperative myocardial ischemia or MI. The incidence of infarction is approximately 5% in large series.35 Preoperative myocardial function and the adequacy of revascularization at surgery should be clear from the history. Determination of circulating levels of CK-MB or troponin postoperatively can provide evidence of perioperative ischemia or infarction.25,26 Often, diminished contractility after operation is caused by inadequate myocardial protection during surgery. Decreased myocardial contractility secondary to inadequate myocardial protection usually resolves within the first 24 hours postoperatively. ECG changes are nonspecific.
Persistent new myocardial dysfunction associated with ECG changes and echocardiographic evidence of new wall-motion abnormalities should raise suspicion that the problem is an occluded graft and MI. Measurements of CK-MB in serum are of limited usefulness because levels of this enzyme are commonly elevated after surgery due to manipulation of the heart and incision of the atria, structures that are rich in the enzyme. If CK-MB levels are very high, greater than 80 mg/dL, then perioperative MI is likely.36 Cardiac troponins are more specific for the diagnosis of perioperative infarction. A comparison of CK-MB, troponin-T, and troponin-I showed that a troponin-I level of greater than 5 µg/L was the most accurate indicator of MI, being superior to either troponin-T or CK-MB.37 Elevated serum concentrations of troponin-I are associated with a cardiac cause of death and with major postoperative complications.38 In addition, troponin-T concentrations measured after surgery are an independent predictor of in-hospital death after cardiac surgery.26 If ischemia or MI is diagnosed, the patient may be taken for angiography or re-exploration and revascularization.
Rate and Rhythm
Bradycardia can lead to ventricular distention, increasing wall tension, and decreasing coronary perfusion pressure, factors that can promote development of ischemia and failure. HR of 80 to 90 appears to be optimal, allowing adequate filling and preventing overdistention but not causing rate-related ischemia. Bradycardia can be corrected by pacing. In general, epicardial pacing wires are left in place after chest closure and are attached to an external pacemaker in the immediate postoperative period. If the dysrhythmia is sinus bradycardia, atrial pacing is usually optimal. The second most common cause of bradyarrhythmia after cardiac surgery is atrioventricular dissociation. The combination of atrial and ventricular leads allows atrioventricular pacing for management of disassociation. Synchronization of the atrioventricular interval between 0.1 and 0.225 second optimizes CO.39
Atrial fibrillation is the most common tachyarrhythmia. It occurs in 10% to 35% of patients after cardiac surgery, usually on the second or third postoperative day. Postoperative atrial fibrillation is associated with increased morbidity and mortality and with longer, more expensive hospital stays.40 The Multicenter Study of Perioperative Ischemia (McSPI) group examined 2417 patients undergoing CABG with or without concurrent valvular surgery.41 The overall incidence of postoperative atrial fibrillation was 27%. Independent predictors of postoperative atrial fibrillation included advanced age, male sex, a past history of atrial fibrillation, a past history of congestive heart failure, and a pre-CPB heart rate greater than 100 beats/min. Surgical practices such as pulmonary vein venting, bicaval venous cannulation, postoperative atrial pacing, and longer cross-clamp times also were identified as independent predictors of postoperative atrial fibrillation. Patients who developed postoperative atrial fibrillation had longer lengths of stay, both in the ICU and in the ward, compared with patients who did not develop the complication.
Although premature ventricular contractions (PVCs) are common, sustained ventricular arrhythmias are far less frequent. Severe ventricular arrhythmias occurring after cardiac surgery are related to ischemia, hypoxemia, hypovolemia, electrolyte abnormalities, the effects of vasoactive drugs, or an underlying preexisting cardiomyopathy.42 In a series of 2100 cardiac operations, only 16 patients (0.8%) developed ventricular fibrillation or a sustained ventricular tachycardia during the interval from 3 days to 3 weeks after surgery. Ten of these patients had undergone valve surgery.43 Prognosis in these patients is dependent on the preoperative ventricular prognosis; it is excellent in those with good function. In those with a left ventricular ejection fraction of less than 40%, the mortality rate may be as high as 75%.44
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree