Fig. 23.1
Incomplete exhalation of delivered tidal volume. Above is a flow volume curve of an asthmatic patient on volume cycled mechanical ventilation. The patient is still exhaling the prior tidal volume at the time of the delivery of the next breath (see arrow). Ideally, the exhalation phase flow rate would return to 0 (complete emptying of tidal volume) prior to delivery of the next breath. The entrapped volume remains in the thorax and can accumulate and cause sequela of air trapping
Over time, air accumulates and becomes “trapped” within the relatively fixed thoracic cage volume. Eventually, residual air increases the intrathoracic pressure (see ideal gas law below for pathophysiologic explanation).
Ideal Gas Law
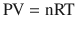
From this relationship, if the amount of gas is increased (n) in a fixed volume (V, in this case the thoracic cage) at a constant temperature, pressure will increase. If left uninterrupted, the increase in thoracic pressure will overcome the venous return pressures in the superior and inferior vena cava. Preload insufficiency and decrease in cardiac output ensue, leading to a state of obstructive shock. This trapped air cannot be discharged while mechanical breaths continue to be administered; such as in this case with the timed volume supported setting of mechanical ventilation. Thus, disconnection of the ventilator tubing from the endotracheal tube and allowing the trapped air to passively escape via an open endotracheal tube remedies this emergency.
The presence of clinically significant air trapping can be determined by measuring the pressure at airway opening (i.e. the pressure detected in the ventilator) at the end of exhalation. The total positive end expiratory pressure, or PEEP, which is composed of the PEEP set on the mechanical ventilator in combination with the pressure exerted by trapped air, or “auto-PEEP”.
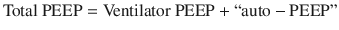
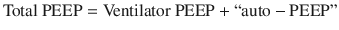
Any pressure measured above the ventilator set PEEP is evidence for some degree of air trapping.
Auto peep also elevates the pressure at airway opening during an end inspiratory hold (plateau pressure) by the below formula (simplified from Truwit et al. [1]).
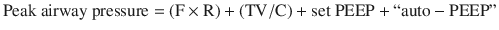
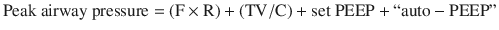
Furthermore, the volume of trapped air can be directly measured and is a sensitive indicator for risk of hypotension from air trapping [2] (see section “Monitoring for Hyperinflation” and Fig. 23.2).
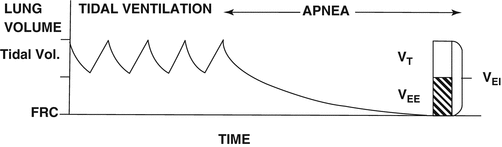

Fig. 23.2
Air trapping during mechanical ventilation in an asthmatic patient. During tidal ventilation, the lung volume never returns its physiologic starting point, the FRC. Measurement of the volume of trapped air (VEE) has been studied in asthmatic patients while receiving mechanical ventilation and pharmacologic paralysis. At the end of a mechanical tidal breath, the respiratory rate is set to zero and the volume of expired air is measured until flow reaches zero. Williams and colleagues have shown that neither barotrauma nor hypotension occur when the volume of trapped air is less than 1.4 L or 15 cc/kg [2]. Note this has only been verified in the paralyzed patient. FRC functional residual capacity, V EE end-expiratory lung volume above FRC (i.e. the volume of trapped gas), V EI end inspiratory lung volume, V T tidal volume (Reprinted with permission of the American Thoracic Society. Copyright © 2015 American Thoracic Society. Williams et al. [2]. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society)
Air trapping can be avoided by minimizing the tidal volume and/or increasing the gas flow rate from the ventilator. Additionally, minute ventilation can be further decreased by reducing the delivered respiratory rate, which may require heavy sedation or neuromuscular blockade. Any decrease in the amount of air that needs to be exhaled, or increase in the amount of time available for exhalation, is useful. When resuming mechanical ventilation in this patient after discharge of the trapped air, she should be treated with substantially lower minute ventilation either by a decreased respiratory rate and/or tidal volume.
Principles of Management
Inhaled Bronchodilators
Inhaled short acting bronchodilators are the mainstay of treatment of an asthma exacerbation. Most commonly, inhaled short acting beta-2 agonists such as albuterol, levalbuterol, or salbutamol are employed. These medications target the underlying physiologic cause of respiratory failure, bronchospasm. They do not, however, treat the underlying inflammatory insult which causes bronchospasm. Albuterol is the most commonly used short acting bronchodilator in the United States. In the most serious cases, it can be used as a continuous nebulized inhaled solution. No data exists in regards to withholding short acting bronchodilators during an asthma exacerbation and equipoise does not exist for such study given the presumed obvious benefit. A meta-analysis showed no advantage of continuous administration of beta agonists, as compared to intermittent administration, in acute asthma [3]. Likewise, a controlled trial in the subset of severe asthma failed to show benefit of continuous beta agonists as compared to treatments every 20 min followed by treatments every hour [4]. For the even smaller subset of life threatening asthma, no prospective data exists in adults to our knowledge.
Addition of inhaled short acting anticholinergic medications to administration of short acting beta agonists improve pulmonary mechanics [5] as well as decrease admission rates [6] in patients with severe asthma, and are recommended by a panel of experts [7]. No data exists in patients receiving mechanical ventilation, though use of anticholinergic may theoretically decrease hypersecretion of mucus and mucus plugging, a finding common in fatal asthma [8].
Corticosteroids
Corticosteroids are administered during an asthma exacerbation to decrease the inflammation that leads to bronchospasm. Meta-analysis has shown a benefit of steroid therapy in adults with acute asthma [9]. No good data exist for the optimal dose of corticosteroids, but 2 mg/kg of methylprednisolone or †he equivalent is recommended by an expert panel [7]. In a randomized trial, no benefit was demonstrated with high dose (500 mg methylprednisolone) versus standard dose (100 mg methylprednisolone) corticosteroids [10].
Monitoring of Arterial Blood Gases
Arterial blood gases are monitored during status asthmaticus in the spontaneously breathing patient. In a patient with adequate respiratory reserve during an asthma exacerbation, an arterial blood gas typically shows respiratory alkalosis. A normal pH and partial pressure of carbon dioxide with a high work of breathing, respiratory acidosis, or normalization of the pH after an initial respiratory alkalosis are all harbingers of impending respiratory embarrassment, and escalation of support with adjunctive treatments and/or invasive mechanical ventilation should be initiated.
Ventilator Strategies
Strategies for asthmatic patients on mechanical ventilation hinge on the avoidance of hyperventilation, or air trapping, as illustrated in our case. Indeed, hypotensive and mechanical complications are related to the volume of gas enclosed in the thorax above functional residual capacity (FRC) at end exhalation [2] (please see section “Monitoring for Hyperinflation”). Entrapment of supra-physiologic gas volumes is best avoided by allowing for full exhalation of tidal volumes and return to FRC. Unfortunately, with severe airway obstruction the exhalation time required to fully empty the lung to FRC can be extremely prolonged. To allow full exhalation, clinicians can either decrease the tidal volume of the inspired breath, or allow additional time for exhalation. In other words, maximization of expiratory time is key. Whatever isn’t inspiratory time is expiratory time.
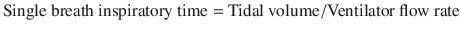
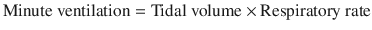
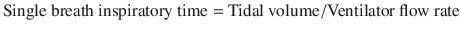
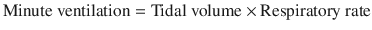
To this end, clinicians can decrease tidal volume in order to decrease the air volume that needs to be exhaled, or decrease the respiratory rate, which increases the time of exhalation. Adjustment of the ratio of inspiratory time to expiratory time by adjusting the flow rate (i.e. 60–100 L/min) or shape (accelerating to square) also increase the amount of exhalation time, however the incremental benefit as compared to decreasing the respiratory rate is minimal at low tidal volumes [11]. Increased flows can also increase spontaneous respiratory rates in some mechanically ventilated patients, which would outweigh any incremental benefit [12]. As a starting setting, a minute ventilation of 10 L per minute or less and a respiratory rate of 10–14 breaths/minute are reasonable [13]. Vigilant monitoring for the efficacy of ventilator settings is needed and is discussed in the section “Monitoring for Hyperinflation”.
Permissive Hypercapnia/Hypoventilation
During permissive hypoventilation, hypercarbia often develops as the minute ventilation provided is not adequate to eliminate the produced carbon dioxide. In a patient with spontaneous respiration on the ventilator, hypercarbia will often lead to an increased respiratory rate, which may be detrimental (see ventilator strategies). Often, deep sedation or paralysis is needed to allow for permissive hypercapnia (or permissive hypoventilation). Elevation in carbon dioxide on arterial blood gas should be tolerated, with a goal arterial blood pH above 7.15 [14]. If the pH drops below 7.15, sodium bicarbonate or THAM infusions can be utilized. Minute ventilation can be increased cautiously if there is no significant hyperinflation. If life-threatening changes in pH continue, further strategies include deeper sedation or paralysis to minimize carbon dioxide production by muscular tissues. In the rare case that these treatments are inadequate, extracorporeal life support (ECLS) can be utilized for CO2 removal (see evidence contour).
Monitoring for Hyperinflation
A thorough understanding of respiratory pressures generated during mechanical ventilation is requisite to understand the pathophysiology of asthma during mechanical ventilation. Peak airway pressure is the combination of several components, as discussed below. These pressures can ONLY be measured in a mode with constant tidal volumes (i.e. not a pressure mode).
Determination of Components of Peak and Plateau Airway Pressure in the Absence of Patient Effort [1]
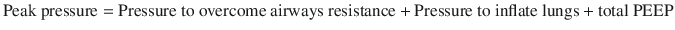
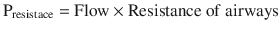
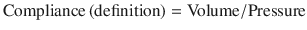
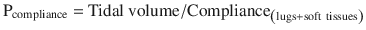
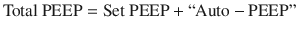

During an inspiratory hold at the end of a full tidal volume (plateau pressure), the flow is zero, eliminating the first term in the equation so
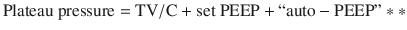 Thus
Thus
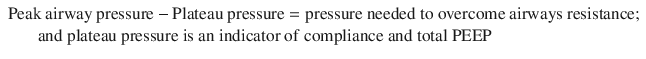
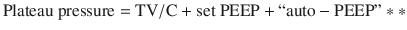
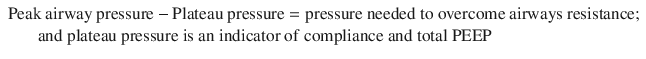
Respiratory causes of death from status asthmaticus often stem from circulatory collapse or mechanical complications of invasive mechanical ventilation, which are typically caused by air trapping and pneumothorax respectively. Avoidance of these complications requires monitoring for hyperinflation. Indeed, the volume of trapped air above functional residual capacity (FRC) at end exhalation correlates with risk for pneumothorax and hypotension (see Fig. 23.1) [2]. However, measuring this volume in clinical practice is difficult due to lack of familiarity with the technique needed (see Fig. 23.2 for full discussion).
Plateau pressure, Auto-PEEP, and analysis of flow volume curves are used as surrogates to directly measuring the entrapped air volume above FRC. Interestingly, plateau pressure and Auto-PEEP did not correlate with barotrauma or hypotensive complications in a single study of mechanically ventilated asthmatic patients [2], despite having some correlation with end inspiratory lung volume (VEi) [15]. However, monitoring of these parameters is advocated by experts as a surrogate for direct measurement of trapped air volume, with goals being auto-PEEP as low as possible and plateau pressures less than 30 cm H2O [11, 16].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







