Fig. 53.1
PA CXR
Fig. 53.2
Lateral CXR
Question
What approach should guide this patient’s treatment of septic shock?
Answer
Six Hour Sepsis Bundle
The management of patients with severe sepsis and septic shock has evolved into protocols which utilize “bundles.” “A bundle is a selected set of elements of care distilled from evidence-based practice guidelines that, when implemented as a group, have an effect on outcomes beyond implementing the individual elements alone” [1]. The Surviving Sepsis Campaign provided the first bundled care process for the management of patients with severe sepsis and septic shock [2]. The first bundle involves care for the initial 3 h of sepsis recognition. The second bundle within the first 6 h of therapy, usually in an intensive care unit (ICU).
This patient was initially given a fluid challenge of 30 mL/kg of 0.9 % normal saline (NS). Blood, sputum, and urine cultures were sent. He was started on broad spectrum antibiotics; Vancomycin, Piperacillin/Tazobactam, and Azithromycin. Despite additional volume administration, his blood pressure failed to normalize after 5 L of 0.9 % NS. A central line was placed and he was started on Norepinephrine. Gram stain from his sputum culture was positive for gram positive cocci in pairs which eventually speciated to Streptococcus pneumonia. Lactic acid level decreased to 3 mmol/L after 6 h and normalized by the next day. Norepinephrine was discontinued after 12 h. The patient received at total of 10 L of 0.9 NS. He was transitioned to a general care floor after 36 h in the ICU. After 48 h, the Streptococcus proved to be pan-sensitive and his antibiotic regimen was changed to Ceftriaxone.
Principles of Management
Definition and Recognition
Despite the advances in modern medicine, sepsis continues to be a leading cause of death and a significant inpatient cost. The incidence of severe sepsis in the United States has been reported to be as high as 300 cases per 100,000 population with an annual cost of $14 billion per year. Sepsis is now defined as, “A life-threatening organ dysfunction due to a dysregulated host response to infection.” Simply stated, sepsis is a suspected infection in the presence of organ dysfunction, where organ dysfunction is defined as in increase in sepsis organ failure assessment (SOFA) score of at least two points.
New Definitions and Constructs
Sepsis is defined as life‐threatening organ dysfunction due to a dysregulated host response to infection
Organ dysfunction can be identified as an acute change in total SOFA score ≥2 points
In lay terms, sepsis is a life-threatening condition that arises when the body’s response to an infection injures its own tissues and organs
Adult patients with suspected infection who are likely to have a prolonged ICU stay or to die in hospital can be promptly identified using qSOFA, i.e., alteration in mental status, systolic blood pressure ≤100 mmHg or respiratory rate ≥22 breaths/min
Septic shock is a subset of sepsis where underlying circulatory and cellular/metabolic abnormalities are profound enough to substantially increase mortality
Adult patients with septic shock can be identified using a clinical construct of sepsis with persisting hypotension requiring vasopressors to maintain MAP ≥65 mmHg, AND a blood lactate >2 mmol/l despite adequate volume resuscitation
Septic shock is now defined as, “a subset of septic patients where underlying circulatory and cellular-metabolic abnormalities are profound enough to substantially increase mortality,” and is characterized by:
The requirement vasopressors to maintain mean BP > 65 mmHg
A lactate > 2 mmol/l
The absence of other causes, especially hypovolemia
The new definitions no longer utilize the term, “severe sepsis.” Additionally the systemic inflammatory response syndrome (SIRS) no longer is part of the definition. The Quick Sepsis Organ Failure Assessment Score (qSOFA) has been found to represent the best screening tool to identify infected patients who are severely ill and at high risk of deterioration:
Glasgow Coma Score ≤ 13
Respiratory rate ≥ 22 breaths/min
Blood pressure ≤ 100 mmHg
This tool works best for patients outside of the intensive care unit. It is meant as a screening tool, instead of SIRS, and is not meant to define sepsis. While the addition of lactate levels failed to improve the qSOFA tool, the addition of other clinical criteria such as lactate can be important in the overall assessment of whether an infected patient requires intervention.
Surviving Sepsis Campaign Sepsis Bundle
Rivers et al. developed a protocol designed to guide the management of severe sepsis and septic shock [5]. The protocol included specific target values for central venous pressure (CVP), mean arterial pressure (MAP), systemic oxygen consumption using mixed venous oxygen saturation (SvO2), hematocrit, cardiac index, and urine output. In response to accumulated evidence the Surviving Sepsis campaign currently lists only mean arterial pressure 65 mmHg as a hemodynamic goal [6]. The 3 h and 6 h bundles are displayed below. The 3 h bundle emphasizes timely administration of broad spectrum antibiotics and an initial fluid administration of 30 ml/kg of crystalloid in the face of an elevated lactate level. The 6 h bundle provides guidance regarding the potential ongoing need for vasopressor administration and/or additional volume administration in the face of ongoing hypotension.
Surviving Sepsis Campaign Bundle [1]
TO BE COMPLETED WITHIN 3 HOURS OF TIME OF PRESENTATION*:
- 1.
Measure lactate level
- 2.
Obtain blood cultures prior to administration of antibiotics
- 3.
Administer broad spectrum antibiotics
- 4.
Administer 30 ml/kg crystalloid for hypotension or lactate ≥4 mmol/L
* “Time of presentation” is defined as the time of triage in the emergency department or, if presenting from another care venue, from the earliest chart annotation consistent with all elements of severe sepsis or septic shock ascertained through chart review
TO BE COMPLETED WITHIN 6 HOURS OF TIME OF PRESENTATION:
- 5.
Apply vasopressors (for hypotension that does not respond to initial fluid resuscitation) to maintain a mean arterial pressure (MAP) ≥65 mmHg
- 6.
In the event of persistent hypotension after initial fluid administration (MAP < 65 mmHg) or if initial lactate was ≥4 mmol/L, re-assess volume status and tissue perfusion
- 7.
Re-measure lactate if initial lactate elevated.
Fluid Management
An important focus of initial treatment in septic shock is sufficient and appropriate early administration of intravenous fluids. Current guidelines recommend crystalloids as the initial fluid choice, with a minimal initial challenge of 30 mL/kg [6]. Nevertheless, albumin is a colloid frequently used for resuscitation in hypotensive patients. The Saline versus Albumin Fluid Evaluation (SAFE) trial compared resuscitation strategies using 4 % albumin or normal saline in all ICU patients and demonstrated similar outcomes [7]. Two studies have compared saline and albumin administration as resuscitation fluid in septic patients. Both showed no difference in 28 day mortality but one study (CRISTAL) did demonstrate improved survival at 90 days in the albumin group [8]. Normal saline is the most commonly used crystalloid. Administration in the setting of sepsis resuscitation has been associated with hyperchloremic (nonanion gap) metabolic acidosis, which had been considered to pose a risk of acute kidney injury [9, 10]. However the use of a chloride-restrictive fluid strategy using a buffered saline solution did not result in a decreased rate of AKI compared with saline when used for resuscitation in a large randomized trial of intensive care patients [11]. Semisynthetic colloids such as hydroxyethyl starch are not recommended for acute resuscitation. In some studies their use has been associated with renal failure, coagulopathy and increased mortality [9, 12]. Blood transfusions were theorized to improve oxygen carrying capacity and improved oxygen delivery. The Rivers EGDT protocol included a hematocrit goal of 30 % or more if, after the improvement of blood pressure, the SvO2 remained less than 70 % [5]. This provision was included in the initial Surviving Sepsis guidelines. In the absence of evidence that transfusions were truly beneficial and with increasing evidence for harm secondary to arbitrary transfusion thresholds, the recommendations were modified to have a goal range of 7–9 g/dl unless myocardial ischemia, coronary artery disease, acute hemorrhage, or severe hypoxemia are present [2, 6, 13, 14].
Hemodynamic Response
Presently the Surviving Sepsis Campaign recommends either a repeat focused physical exam (including vital signs, capillary refill, pulse and skin findings) or the measurement of two of the following parameters: central venous pressure; superior vena cava oxygen saturation; bedside cardiovascular ultrasound; or dynamic assessment of fluid responsiveness with passive leg raise or fluid challenge.
Skin mottling has been described in sepsis for over 60 years [15]. Increasing evidence has identified a crucial role of microcirculation impairment in severe infections. One center developed a mottling score (from 0 to 5), based on mottling area extension from the knees to the periphery. There was improved survival in patients whose mottling score decreased during the resuscitation period [16] (Fig. 53.3).
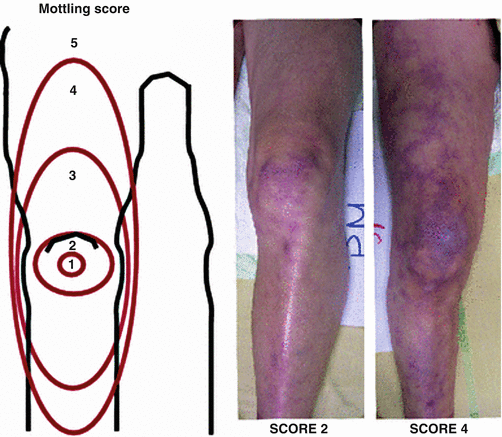

Fig. 53.3
Skin Mottling Score. Left: the mottling score is based on a mottling area extension on the legs. Score 0 indicates no mottling; score 1, a modest mottling area (coin size) localized to the center of the knee; score 2, a moderate mottling area that does not exceed the superior edge of the kneecap; score 3, a mild mottling area that does not exceed the middle thigh; score 4, a severe mottling area that does not go beyond the fold of the groin; score 5, an extremely severe mottling area that goes beyond the fold of the groin. Right: Examples of the mottling score
Central venous pressure (CVP) remains a common approach to monitor fluid responsiveness but should be used in conjunction with other dynamic variables. The goal has been 8–12 mmHg for non-ventilated patients and 12–15 mmHg for those requiring mechanical ventilation [17]. Since CVP may not accurately assess volume status especially in mechanically ventilated patients with high airway pressures, measurement of CVP should not be used in isolation.
Mixed venous oxygen saturation measures the net balance between oxygen delivery (influenced by arterial oxygen saturation, hemoglobin, and cardiac output) and oxygen consumption by the tissues. Samples obtained from the subclavian or vena cava typically show higher saturation than samples from the right atrium. The target for adequate venous oxygenation is 70 % or more for superior vena cava and 65 % for the right atria [17]. Pulmonary arterial catheterization and monitoring have not shown to improve outcomes. The procedure may also confer risk to the patient.
Passive leg raise is a simple method of assessing intravascular volume and fluid responsiveness. The patient is moved to a supine position with the legs raised to 45° for several minutes. An increase of venous return causing a ≥ 10 % increase in aortic blood flow measured by esophageal Doppler and arterial pulse pressure signaled a response to fluids [18]. Dynamic variables such as pulse pressure variation or ultrasound evaluation of the inferior vena cava (IVC) can also be used. Pulse pressure variation is calculated via arterial line measurements of maximum and minimum pulse pressure during a single respiratory cycle (Fig. 53.4). Increasing variation predicts fluid responsiveness [19].
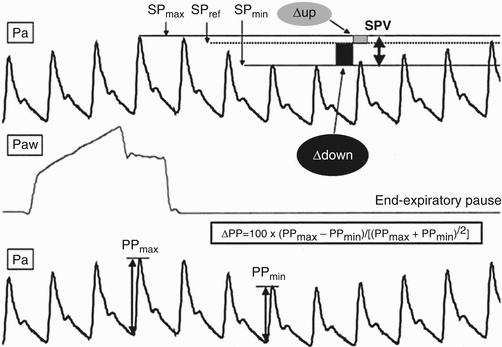

Fig. 53.4
Pulse pressure variation. Analytical description of respiratory changes in arterial pressure during mechanical ventilation. The systolic pressure and the pulse pressure (systolic minus diastolic pressure) are maximum (SPmax and PPmax, respectively) during inspiration and minimum (SPmin and PPmin, respectively) a few heartbeats later, i.e., during the expiratory period. The systolic pressure variation (SPV) is the difference between SPmax and SPmin. The assessment of a reference systolic pressure (SPref) during an end-expiratory pause allows the discrimination between the inspiratory increase (Δup) and the expiratory decrease (Δdown) in systolic pressure. Pa arterial pressure, Paw airway pressure (From Michard et al. [54]. Reprinted with permission from Wolters Kluwer Health, Inc)
Focused ultrasonography is another method to discern central hemodynamics and the etiology of shock. It can reveal right and left cardiac chamber size and contractility, pericardial fluid, and inferior vena cava size and collapsibility suggestive of hypovolemia, among other features. A minimally collapsible IVC is associated with euvolemia or hypervolemia while a highly collapsible IVC is associated with hypovolemia [20] (Video 53.1). Recent guidelines and consensus statements recommend focused ultrasonography as best clinical practice in the initial assessment of hemodynamically unstable patients with septic shock despite no rigorous RCTs of focused cardiac ultrasonography affecting patient-centered outcomes in septic shock [21].
Tissue Perfusion
Elevated lactate level > 2 are part of the diagnostic criteria for septic shock, although the Surviving Sepsis Campaign has previously utilized a level of 4. Lactate clearance is used as a marker of improving tissue perfusion and is a target of early therapy. The goal is normalization of lactate, however early improvement of at least 10–20 % from baseline lactate is associated with a mortality benefit comparable to a SvO2 of 70 % or more [22]. The rigorous targeting of SvO2 to over 70 % is not essential for the early management of sepsis. SvO2 is now considered one of several methods of evaluating successful resuscitation. Strategies to optimize both lactate clearance and SvO2 may be complimentary and currently, no single measure is clearly superior.
Vasopressors
Norepinephrine is recommended as the first line agent for use in patients with septic shock. Dopamine is associated with a higher rate of dysrhythmias and low-dose dopamine for renal protection is not recommended [23]. Vasopressin at .01 to .03 U per minute was compared to norepinephrine in the VASST study [24]. There was no significant mortality difference at 28 or 90 days in all patients with sepsis. The Surviving Sepsis Guidelines states that Vasopressin at .03 U/min can be used in an effort to further raise mean arterial pressure (MAP) or to decrease the dose of norepinephrine. It is not recommended as a first line agent. There have been few studies evaluating phenylephrine, an ὰ-1 adrenergic receptor agonist, in sepsis. It is also not recommended as a first line agent.
Treatment of Infection and Antimicrobial Stewardship
Source control is defined as “all physical measures undertaken to eliminate a source of infection, to control ongoing contamination, and to restore premorbid anatomy and function” [25]. The Surviving Sepsis Guidelines define source control as: a) specific diagnosis of infection and intervention within 12 h of diagnosis, b) if infected peripancreatic necrosis is identified, definitive intervention is best delayed until adequate demarcation of viable and nonviable tissues has occurred, c) effective intervention associated with the least physiologic insult should be used (e.g., percutaneous rather than surgical drainage of an abscess), and d) if intravascular access devices are a possible source of sepsis or septic shock, they should be removed promptly after other vascular access has been established [6].
The initial intravenous antimicrobial therapy should have broad coverage and adequate tissue penetration against all likely pathogens. Therapy should be given within the first hour after the recognition of sepsis or septic shock. Mortality increases for each hour that the patient does not receive adequate antimicrobial therapy while the patient is hypotensive [26]. Two sets of blood cultures along with cultures from other potential sources such as urine or sputum should be obtained before the initiation of antimicrobials if it can be done without significant delay. With the mortality risk associated with a delay in therapy, the initiation of empiric, broad-spectrum antimicrobials has become a recommendation within the six hour window. A side-effect of this practice may be increased antimicrobial resistance which can potentially result in a prolonged hospital stay with a less clear effect on mortality [4]. Daily assessment is important for potentially de-escalating or modifying therapy [27, 28].
Ventilator Management
In mechanically ventilated patients, lung-protective ventilation is recommended in patients with severe sepsis or septic shock regardless of whether or not the patient has been diagnosed with acute respiratory distress syndrome. A tidal volume of 6 mL/kg of ideal body weight should be combined with a goal plateau pressure <30 cm H2O along with the application of positive end-expiratory pressure [6, 29, 30].
Glycemic Control and Nutrition
Glucose control in the critically ill patient has evolved significantly over the years. An early study showed improved outcomes and fewer complications with glucose maintained at approximately 80–108 mg/dL. This was especially true in surgical patients [31]. The NICE-SUGAR trial found that tight glycemic control was associated with higher 90 day mortality [32]. The higher mortality in NICE-SUGAR was accounted for by septic patients and therefore a glucose level of 80–110 mg/dL is considered contraindicated in septic patients who are being resuscitated. Current guidelines call for glucose monitoring and management with insulin after 2 consecutive blood glucose values are more than 180 mg/dL. The goal is to maintain a level at 180 mg/dL or less without a lower target other than hypoglycemia [6].
There is little evidence of benefit to starting full enteral or parenteral nutrition early in the course of severe sepsis. Enteral feeding may be initiated after the initial resuscitation, if tolerated. Parenteral feeding should not be provided within the first week and should be avoided if enteral feeding is possible. There is an association with improved outcomes including mortality with hypocaloric feeding when initiated within the first week [6, 33].
Evidence Contour
The EGDT bundle has remained one of the cornerstones in the management of severe sepsis and septic shock. Subsequently, multiple studies have analyzed each component of the bundle which has resulted in an evolution of practice. Those changes are represented in the most recent Surviving Sepsis Campaign guidelines.
Early Goal Directed Therapy (EGDT)
The Protocolized Care for Early Septic Shock (ProCESS) trial prospectively randomized 1341 patients in a 1:1:1 ratio into 1 of 3 groups. One group was a protocol based EGDT group which followed the initial study protocols. The second group was a protocol based therapy that required rapid resuscitation but no requirement for initial central line placement, mixed venous oxygen saturation (SvO2) monitoring, or blood transfusions for a hematocrit <30. The third group was a “usual care” arm in which care was directed by the bedside clinician. The protocol-based EGDT did not require additional organ support i.e., dialysis or mechanical ventilation or demonstrate any improvement in 2–3 month or 1 year mortality compared to the other 2 arms [34]. Multiple multi-center studies resulted in similar findings. The ARISE trial randomized 1600 patients into an EGDT and a usual care group. The EGDT group did not reduce all-cause mortality at 90 days. There was no significant difference in in-hospital mortality, duration of organ support or length of hospital stay [35]. 1260 patients were enrolled in the ProMISe trial. 630 patients were assigned to the EGDT arm and 630 were assigned to “usual care”. There was no difference in all-cause mortality at 90 days [36]. The mortality associated with sepsis from all arms from the first 2 trials ranged from 18.2 to 21 % while the mortality from the ProMISe trial was 29.5 % in the EGDT group and 29.2 % in the usual care group. In all likelihood that “standard” care for sepsis has improved and evolved since the original publication of EGDT [37]. A retrospective, observational review of >100,000 patients with severe sepsis from Australia and New Zealand from 2000 to 2012 demonstrated improved mortality [38]. The authors attributed part of the improvement on overall changes in ICU practice. The focus on sepsis treatment remains with early fluid resuscitation, timely antibiotic administration, and appropriate use of vasopressors (Table 53.1).
Table 53.1




Guidelines for the treatment of sepsis and septic shock from the Surviving Sepsis Campaign
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







