205 Management of Pain, Anxiety, and Delirium
Pain, anxiety, and delirium are extremely common in the ICU, where they are often underappreciated and inadequately treated. However, unrelieved pain, anxiety, and delirium contribute to patient distress, evoke the stress response, complicate the management of lifesaving devices, and negatively affect outcome. Ensuring patient comfort and safety is a universal goal that has been endorsed by national medical societies and oversight bodies such as the Center for Medicare and Medicaid Services and The Joint Commission (TJC), which accredits and certifies U.S. healthcare organizations and programs.1 Regional preferences, patient history, institutional bias, and individual patient variability, however, create a wide discrepancy in the approach to sedation of critically ill patients.
 General Principles
General Principles
Sedation and analgesia are administered to provide comfort and ensure patient safety, especially during mechanical ventilation. As a first step, it is important that the healthcare provider evaluate the specific problem requiring sedation to then devise an appropriate treatment strategy. Routine and objective assessments using valid and reliable measures of pain, anxiety, and delirium are vital. Scales to measure these conditions provide a common language for providers to use in quantifying their degree and recording the patient’s response to therapy. It is important to frequently reassess and adjust therapeutic targets based on the condition of the patient. Pain must always be addressed first; unrelieved pain can be the underlying cause of anxiety, agitation, and delirium. Once pain is adequately controlled, anxiety should then be treated with an anxiolytic or sedative. In critically ill patients, unpredictable pharmacokinetics and pharmacodynamics secondary to drug interactions, organ dysfunction, absorption, protein binding, and hemodynamic instability can lead to medication complications.2 Because most of these agents are administered as continuous infusions, drug accumulation, redistribution, and tachyphylaxis also confound their use, and techniques to prevent systemic drug accumulation have to be employed.
 Pain
Pain
Existing disease, surgical procedures, trauma, invasive monitors, endotracheal intubation, and nursing interventions are only a few sources of discomfort commonly experienced by patients in the intensive care unit (ICU). In addition to patient discomfort, inadequately treated pain leads to an increased stress response, with resultant tachycardia, increased oxygen consumption, hypercoagulability, immunosuppression, hypermetabolism, and increased endogenous catecholamine activity.2–5 Insufficient pain relief can also contribute to deficient sleep, disorientation, and anxiety, and long-term effects such as posttraumatic stress disorder may also be seen.6 Unfortunately, pain is often undertreated because of concerns about the adverse effects and addiction potential of opiates and because caregivers lack the necessary skills for proper pain assessment and treatment.3
Assessment of Pain
To be recognized and properly treated, pain must be routinely and objectively assessed. In the ICU, the most valid and reliable indicator of pain is the patient’s self-report.7 Information about pain including location, quality, and intensity should be elicited as part of routine checking and recording of the patient’s vital signs. Intensity can be objectively measured using tools such as the visual analog scale or numeric rating scale.7
It is not uncommon for ICU patients to be unable to communicate with caregivers owing to endotracheal intubation or altered mental status. During such times, behavioral and physiologic indicators must be used to assess pain intensity. The FACES scale8 (Figure 205-1) was developed to objectify the use of facial expression as a measure of pain intensity and shows moderate correlation with different levels of pain.9 Unfortunately, these indicators are nonspecific and subjective in nature, and as a result, clinicians are likely to underestimate and undertreat pain. The Behavioral Pain Scale (Table 205-1) is a valid tool that uses facial expression, limb movements, and ventilator synchrony for calculating a pain score; use of such pain assessments has been associated with lower analgesic and sedative use and with decreased time on the ventilator.10,11
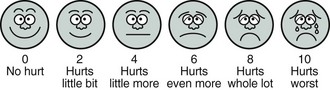
Figure 205-1 FACES scale.
(From Wong DL, Hockenberry-Eaton M, Wilson D et al. Wong’s essentials of pediatric nursing. 6th ed. St Louis: Mosby; 2001, p. 1301.)
TABLE 205-1 The Behavioral Pain Scale
| Item | Description | Score |
|---|---|---|
| Facial expression | Relaxed | 1 |
| Partially tightened (e.g., brow lowering) | 2 | |
| Fully tightened (e.g., eyelid closing) | 3 | |
| Grimacing | 4 | |
| Upper limbs | No movement | 1 |
| Partially bent | 2 | |
| Fully bent with finger flexion | 3 | |
| Permanently retracted | 4 | |
| Compliance with ventilation | Tolerating movement | 1 |
| Coughing but tolerating ventilation for most of the time | 2 | |
| Fighting ventilator | 3 | |
| Unable to control ventilation | 4 |
Modified from Payen JF, Bru O, Bosson JL et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med 2001;29:2258-63.
Management of Pain
In managing pain, nonpharmacologic methods should be attempted first. These include patient repositioning, injury stabilization, removal of noxious or irritating stimuli, and application of heat or cold.3 When nonpharmacologic approaches are insufficient to provide analgesia, regional or systemic therapy is indicated.
Regional Therapy
Regional nerve blockade provides analgesia for a large area of the body without the global effects of systemic analgesia. These procedures should be carried out only by clinicians trained specifically in their performance and management. Intercostal blocks can be used to manage pain due to thoracic or upper abdominal trauma or surgery and can improve respiratory mechanics to reduce the risk of pulmonary compromise.12 Intercostal blocks have the advantage of providing analgesia without sedation or respiratory depression. Placement of an intercostal block carries the risk of pneumothorax and may have to be repeated because of its limited duration of action. Paravertebral blocks are useful for managing pain related to unilateral thoracic or abdominal procedures.13 Paravertebral blockade carries the risk of inadvertent epidural blockade, pneumothorax, and hemothorax. Paravertebral blockade has been shown to have equal effectiveness as epidural blockade for pain control in traumatic rib fractures14 but decreased pain control when compared to epidural blockade in thoracic surgery patients.15 Blockade of the brachial plexus, lumbar plexus, sacral plexus, or the individual nerves of these plexuses may prove beneficial for the relief of pain localized to one extremity and can facilitate patient care such as dressing changes, frequent turning, or physical therapy.16 These nerve blocks are generally well tolerated, can be prolonged by the placement of peripheral nerve catheters, and have the benefit of targeted and localized action.
Epidural analgesia has become increasingly popular for the management of pain from thoracic, abdominal, or lower extremity operative procedures.17 Through a catheter, local anesthetics, opiates, and other pharmaceutical adjuncts like clonidine can be infused in the epidural space to provide bilateral analgesia in specific dermatomes. Low-concentration bupivacaine (e.g., 0.1%) or ropivacaine (e.g., 0.2%) provides excellent sensory blockade with minimal motor blockade.18 Hypotension from sympathetic blockade and inability to ambulate due to decreased proprioception and/or motor weakness are known side effects.18 Opiates such morphine, hydromorphone, and fentanyl are often added to local anesthetic solutions for their synergistic analgesic effects and do not cause sympathetic or motor blockade.18 Possible adverse effects of epidural opiates include respiratory depression, urinary retention, nausea, vomiting, pruritus, and headache.19 Multiple studies and meta-analyses examining epidural analgesia have shown reduced morbidity after major surgery, including improved pulmonary and intestinal function,17,20 but epidural analgesia has not been shown to reduce mortality or length of stay despite improving pulmonary function in a meta-analysis of traumatic rib fracture patients, a commonly prescribed indication.21 Epidural catheters should be used with extreme caution in patients who are receiving anticoagulation, especially low-molecular-weight heparin, because of the risk of epidural hematoma and paralysis from catheter manipulation.22
Systemic Therapy
The most commonly used opiates in the ICU are morphine, hydromorphone, and fentanyl, though remifentanil is gaining popularity as an ultra short-acting analgesic-sedative drug. All these agents provide less sedation than the commonly used hypnotics or anesthetic agents, and patients receiving analgesic-based regimens with opioids are more likely to have accurate memory and less likely to suffer from posttraumatic stress disorder.23
Morphine is typically used as an intermittent intravenous (IV) injection. With IV injection, its peak effect occurs within 15 to 20 minutes, and analgesia lasts 2 to 4 hours. Morphine is given in doses of 2 to 5 mg IV every 5 to 15 minutes until the pain is controlled, followed by similar doses on a scheduled basis every 1 to 2 hours, with extra doses available as needed for breakthrough pain. Morphine is characterized by hepatic metabolism and renal excretion, and its effects can be prolonged in patients with renal impairment secondary to accumulation of an active metabolite (morphine-6 glucuronide).24
Hydromorphone is a more potent congener of morphine with similar pharmacokinetic and pharmacodynamic profiles.24 Its lack of histamine release and decreased incidence of central nervous system (CNS) side effects make it a useful alternative to morphine, with typical dosing ranges of 0.2 mg to 1 mg IV.
Fentanyl is a synthetic opioid with a rapid onset (5-15 minutes) and a short duration of action (30-60 minutes).24 Because of its short half-life, it can be easily titrated as a continuous infusion. Loading doses of 25 to 100 µg are given every 5 to 10 minutes until the pain is controlled, followed by infusion rates of 25 to 250 µg/h. Because it causes less histamine release than morphine and does not undergo renal elimination, it is the preferred opioid analgesic in hemodynamically unstable patients or those with renal insufficiency.3
Remifentanil is a derivative of fentanyl that is metabolized by nonspecific blood and tissue esterases.24 It is used primarily as an infusion and has an elimination half-life of under 10 minutes regardless of infusion duration.25 Hypotension and bradycardia are the most common side effects of remifentanil administration, and supplemental analgesic medication is usually required at the conclusion of a remifentanil infusion.25
Few comparative trials of opioid infusions have been performed. Traditionally, the selection of an opioid depends on the likely duration of analgesic infusion and the pharmacology of the specific opioid.3 In a randomized double blind study, the mean percentage of hours at optimal sedation was significantly longer for patients receiving remifentanil versus morphine, and the duration of mechanical ventilation and extubation time were shorter for patients receiving remifentanil.26 More patients in the morphine group also required the addition of midazolam for supplemental sedation. When compared with fentanyl, efficacy of achieving sedation goals was similar with remifentanil, though more breakthrough propofol was required in the fentanyl group.27 There were no differences in time to extubation in both groups, but the percentage of patients experiencing pain after extubation was significantly higher in those receiving remifentanil, indicating the need for proactive pain management when weaning remifentanil.
 Anxiety, Agitation, and Sedation
Anxiety, Agitation, and Sedation
Anxiety is a diffuse and unpleasant emotion of apprehension that is not associated with a specific threat. Agitation is a state of anxiety accompanied by extreme arousal, irritability, and motor restlessness. Both are very common in the ICU, where a variety of triggers are responsible: excessive stimulation, pain, dyspnea, delirium, inability to communicate, sleep deprivation, metabolic disturbances, and underlying anxiety disorders. Anxiety can be present without agitation, as evidenced by anxious patients who become fearful and withdrawn. Unrelieved anxiety can be a significant source of physical and psychological stress for patients both during an acute event and in the long term, when unpleasant, frightening memories and posttraumatic stress disorder may result.6,28 Left untreated, agitation can become life threatening if it leads to the removal of lifesaving devices such as endotracheal tubes and intravascular lines. Like pain, anxiety and agitation require a systematic, targeted approach in their assessment and treatment.
Sedation Scales and Protocols
There are many scales available for the assessment of sedation and agitation, including the Ramsay Sedation Scale (RSS),29 the Riker Sedation-Agitation Scale (SAS),30 the Motor Activity Assessment Scale (MAAS),31 and Richmond Agitation-Sedation Scale (RASS).32 Each has good reliability and validity among adult ICU patients and can be used to set targets for goal-directed therapy. However, only the RASS has been shown to detect variations in the level of consciousness over time or in response to changes in sedative and analgesic drug use.3,33 The RASS is a 10-point scale with discrete criteria to distinguish levels of agitation and sedation (Table 205-2) and takes less than 20 seconds to complete. Numerous studies have now shown that the use of a defined sedation target for the provision of protocol-based, goal-directed therapy reduces patient discomfort and improves outcome.32,34,35 Additionally, the use of protocols that incorporate daily interruption of sedation,36 as well as link these spontaneous awakening trials with daily spontaneous breathing trials,37 has been shown to improve time off mechanical ventilation and shorten ICU and hospital stays, without antecedent adverse effects. Furthermore, the Awakening and Breathing Controlled (ABC) Trial showed a reduction in mortality at 12 months by incorporating this linked approach. Neither of these studies found any long-term neuropsychological consequences of performing daily sedation holds.38,39
TABLE 205-2 The Richmond Agitation-Sedation Scale
| Richmond Agitation-Sedation Scale (RASS) | ||
| +4 | Combative | Combative, violent, immediate danger to staff |
| +3 | Very agitated | Pulls or removes tubes or catheters; aggressive |
| +2 | Agitated | Frequent nonpurposeful movement; fights ventilator |
| +1 | Restless | Anxious, apprehensive, but movements not aggressive or vigorous |
| 0 | Alert and calm | |
| −1 | Drowsy | Not fully alert, but has sustained (>10 sec) awakening (eye opening/contact) to voice |
| −2 | Light sedation | Drowsy, briefly (<10 sec) awakens to voice or physical stimulation |
| −3 | Moderate sedation | Movement or eye opening (but no eye contact) to voice |
| −4 | Deep sedation | No response to voice, but movement or eye opening to physical stimulation |
| −5 | Unarousable | No response to voice or physical stimulation |
Adapted from Sessler CN, Gosnell MS, Grapp MJ et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338-44; and from Ely EW, Truman B, Shintani A et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale [RASS]. JAMA 2003;289:2983-91.
Pharmacologic Management
Benzodiazepines bind to γ-aminobutyric acid (GABA) receptors in the CNS, thereby providing sedation, anxiolysis, hypnosis, muscle relaxation, anticonvulsant activity, and amnesia.40 These agents do not relieve pain, but their anxiolytic and amnestic properties may improve pain tolerance by moderating the anticipatory pain response.41 Benzodiazepines vary considerably in their pharmacology, and patient-specific factors such as advanced age, drug or alcohol use, and organ dysfunction make their potency, onset, and duration of action even more unpredictable. When given in bolus doses, these drugs can cause hypotension secondary to decreased sympathetic tone, particularly in hemodynamically unstable patients.40 By reducing inhibitions, benzodiazepines may paradoxically increase agitation and aggressiveness. Benzodiazepines can also cause delirium, so their use in treating hyperactive delirium can be counterproductive. Of the benzodiazepines that are currently available, diazepam, midazolam, and lorazepam are used most frequently in the ICU. The onset of action of diazepam is 2 to 5 minutes, making it useful for rapidly sedating acutely agitated patients. However, its long half-life makes prolonged sedation a risk with repeated use, particularly in patients with renal or hepatic dysfunction.40 To control acute agitation, diazepam is given in doses of 2 to 5 mg IV every 5 to 15 minutes until the event is controlled. Continuous infusions are not recommended.
Midazolam is also useful for acute agitation because it has a rapid onset (2-5 minutes) and a short duration of action.40 It is given as bolus injections of 2 to 5 mg IV every 5 to 15 minutes. When used for long-term sedation (>48 hours), it tends to produce unpredictable awakening times, especially in patients who are obese, have low serum albumin concentrations, or have renal or hepatic failure.3 Lorazepam has a slower onset of action (5-20 minutes), making it less helpful for acute agitation. However, it is less lipid soluble and has no active metabolites, making it potentially useful for long-term administration in critically ill patients.3,40 Intermittent doses of 1 to 4 mg IV are given every 2 to 6 hours, or continuous infusions may be used, although recent data have suggested significant morbidity associated with lorazepam infusions,42 including concerns of propylene glycol toxicity.43
Propofol is an IV anesthetic that acts primarily at the GABA receptor.44 It has proven utility as a sedating agent in the ICU owing to its rapid onset (1-2 minutes) and short duration of action (2-8 minutes). It is typically given as a bolus injection of 40 to 100 mg IV followed by an infusion of 25 to 75 µg/kg/min.45 Propofol is especially useful when rapid awakening is important, such as for neurologic assessment or pending extubation.3 As a respiratory depressant, propofol suppresses both central and peripheral stimuli for ventilation. It can also cause significant hypotension by venodilation, vasodilation, and myocardial depression.46 These cardiovascular effects can be minimized by titration of infusions slowly to achieve the desired sedation level. Propofol has been associated with hypertriglyceridemia when infused for 7 days or longer, leading to the recommendation that infusions should be used at the lowest possible dose for the shortest possible time.3 Another complication associated with propofol use is the development of propofol infusion syndrome, characterized by severe lactic acidosis and rhabdomyolysis.47 Although the majority of reports have been in the pediatric population, a handful of case reports have been published about propofol infusion syndrome associated with high-dose (>75 µg/kg/min) and prolonged (>72 hours) infusions in adults as well.47 Consequently, providers should consider alternative sedative agents for any patient receiving high-dose propofol infusions who develops unexplained metabolic acidosis, arrhythmia, or cardiac failure.
Dexmedetomidine is a selective α2 receptor agonist with a site of action that includes presynaptic neurons in the locus ceruleus and spinal cord; it produces analgesia and sedation without respiratory suppression.48 The onset of action is within 15 minutes, and peak concentrations are achieved after 1 hour of continuous infusion.48 Sedation is often initiated with a bolus of 1 µg/kg over 10 to 20 minutes, followed by an infusion of 0.2 to 0.7 µg/kg/h. Several studies have shown safety with doses up to 2 µg/kg/h, although with increased incidence of bradycardia and hypotension.49 Patients with severe liver disease require lower dosing, whereas dose adjustment is not required in those with renal dysfunction.48 Bradycardia is the most common side effect of dexmedetomidine, especially with rapid bolus administration. A biphasic response in blood pressure may be seen during dexmedetomidine use, with decreased blood pressure at lower concentrations and increased blood pressure at higher concentrations.48 Dexmedetomidine has been shown to attenuate inflammatory responses,50,51 mimic natural non–rapid eye movement sleep,52 and have antiapoptotic actions,53 which may make it an attractive agent for sedation in the ICU, though further studies are warranted to show benefit of these actions.
Multiple studies have been performed comparing different sedative therapies in ICU patients. A study of short-duration sedation (<8 hours) revealed no significant differences between intermittent lorazepam and continuous-infusion midazolam in terms of quality of sedation, anxiolysis, hemodynamic and oxygen transport variables, and patient and nurse satisfaction.54 However, lorazepam was deemed more cost-effective because larger doses of midazolam were required to produce the desired level of sedation. A pharmacologic model comparing lorazepam and midazolam infusions found the emergence times for light and deep sedation to be significantly longer for lorazepam than midazolam.55 In a prospective randomized controlled study in trauma patients comparing infusions of lorazepam, midazolam, and propofol, oversedation occurred most frequently with lorazepam, and the greatest number of dosage adjustments was required by the lorazepam group.56 Undersedation occurred most often with propofol, and this drug had the highest cost of sedation. The study’s data indicated midazolam as the most titratable drug with the least amount of oversedation or undersedation and suggested that lorazepam was the most cost-effective agent for sedation.
Propofol has been compared to individual benzodiazepines in several studies. In a randomized trial comparing intermittent lorazepam boluses to propofol infusion, with daily interruption of sedatives in both groups, patients in the propofol group had fewer mechanical ventilation days, with a trend toward greater number of ventilator-free survival days.57 In an economic evaluation of propofol versus lorazepam, propofol was determined to be less costly per patient than lorazepam despite the considerably lower pharmacy unit cost of lorazepam.58 The lower costs were likely attributable to the greater number of ventilator-free days in patients treated with propofol. Several studies have compared propofol and midazolam infusions.59–61 In a systematic review of these trials, duration of adequate sedation was found to be greater with propofol, independent of length of sedation.62 Weaning times were found to be shorter with propofol, but this was only statistically significant in patients sedated for less than 36 hours. The review surmised that effective sedation was possible with both propofol and midazolam, and it also determined that 1 of 12 patients sedated with propofol was likely to develop hypotension that would not occur with midazolam sedation.
Dexmedetomidine, a newer agent, has subsequently been compared to preexisting sedation regimens. One of the first comparative studies found that patients sedated with dexmedetomidine were adequately sedated and required three times less opiates than patients sedated with propofol.63 Patients on dexmedetomidine had lower heart rates, but there was no difference in arterial blood pressure among the group. Dexmedetomidine has also been studied in patients after coronary artery bypass surgery, with similar times to weaning and extubation in patients treated with dexmedetomidine or propofol, though there was a significant reduction in use of narcotics, beta-blockers, antiemetics, NSAIDs, epinephrine, and diuretics in patients receiving dexmedetomidine.64 One study evaluated patient ratings of sedation during mechanical ventilation and found that patients on dexmedetomidine perceived a shorter length of intubation despite no actual difference in length of intubation or length of ICU stay.65 A double-blind randomized controlled trial (the MENDS study) comparing sedation with dexmedetomidine to lorazepam in mechanically ventilated surgical and medical ICU patients found that sedation with dexmedetomidine resulted in more days alive without delirium or coma, lower prevalence of coma, greater achievement of target sedation, and minimal differences in cost of care despite the higher acquisition cost of dexmedetomidine.66 A further analysis of these patients revealed improvements in daily delirium rates in the dexmedetomidine group.67 Patients with sepsis who were sedated with dexmedetomidine had shorter time on mechanical ventilation and improved survival when compared to the lorazepam group, without any differences in hemodynamic profiles or adverse events.67 Another multicenter double-blind randomized trial (the SEDCOM study) comparing dexmedetomidine with midazolam sedation found no difference in time at targeted sedation level, but patients treated with dexmedetomidine spent less time on the ventilator, experienced less delirium, and developed less tachycardia and hypertension.68 Finally, a meta-analysis suggested that sedation with dexmedetomidine decreases ICU length of stay.49
The safety and efficacy of analgesia-based sedation with remifentanil has been compared to conventional sedation with hypnotic-based regimens for patients with brain injury requiring prolonged sedation for mechanical ventilation.69 Neurologic assessment times and time to extubation were significantly shorter for patients receiving remifentanil than those receiving propofol or midazolam supplemented with morphine or fentanyl. In another study comparing remifentanil-based sedation with a midazolam-based regime, the duration of mechanical ventilation and duration of weaning were significantly shorter in patients receiving remifentanil, and a trend toward shortened ICU stay was also observed.70 A randomized multicenter study comparing conventional sedation regimens (propofol or benzodiazepine with as-needed opioid) with an analgesia-based regimen consisting of remifentanil with as-needed propofol found shortened durations of mechanical ventilation and ICU length of stay in the analgesia-based group.71 However, concerns about costs, withdrawal, and hyperalgesia after discontinuation of remifentanil have limited widespread use of this agent in the United States.72 A recent single-center randomized controlled study compared the use of an analgesia-based protocol incorporating morphine (intervention group) to sedation with propofol.73 Patients in the intervention group had shorter times on mechanical ventilation and in the ICU, with no adverse events. About 20% of the patients in the “no sedation, morphine only” group required rescue with propofol per the protocol; however, 80% were managed with morphine alone despite being critically ill.73 The generalizability of this study is limited by the fact that the ICU had 1 : 1 nursing ratios, as well as other personnel to help reassure patients, which may not be available in most other ICUs.
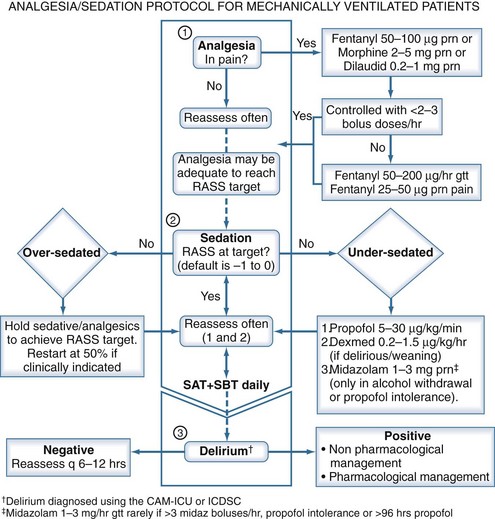
An empirical protocol for the management of pain, anxiety, and sedation is provided as a reference in Figure 205-2. Readers are advised to incorporate local culture, patient characteristics, and expert opinion to determine the best protocol for their respective ICUs.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree