CHAPTER 90 MANAGEMENT OF ENDOCRINE DISORDERS IN THE SURGICAL INTENSIVE CARE UNIT
The endocrine system as a part of the neuroendocrine axis (hypothalamic-pituitary axis) influences the response to stress and critical illness. Endocrine abnormalities within this axis change and modify the physiologic response to trauma and stress. Critically ill patients with a known diagnosis of an endocrine problem are treated with replacement therapy. However, an unrecognized endocrine abnormality often creates management difficulties and increases morbidity. Endocrine problems occur at all levels of the neuroendocrine axis from primary or secondary disease, medications, or end-organ failure.
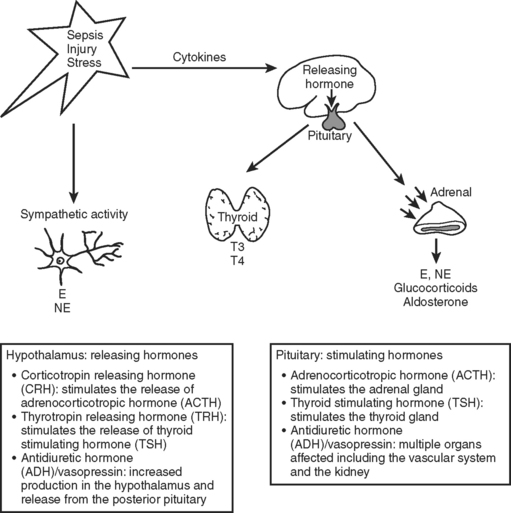
The neuroendocrine axis is responsible for the stress response and is controlled by the hypothalamus, pituitary, and the autonomic nervous system (Figure 1). This axis is activated by baroreceptor response to intravascular volume, sympathetic response from tissue injury, and inflammatory mediators released from tissue trauma. The hormones released in response to injury act through binding to cell surface receptors or intracellular receptors and produce a complex series of responses and feedback loops that maintain cellular processes.1 This chapter addresses abnormalities in the endocrine system that affect the course of critically ill patients.
BRAIN PROBLEMS: ABNORMALITIES IN HYPOTHALAMIC/PITUITARY RESPONSE
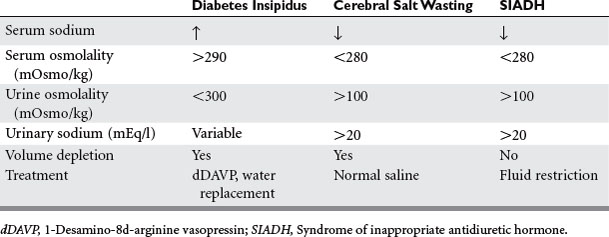
Injuries that affect the brain can interrupt the hypothalamus or pituitary production of hormone. Head injury, brain surgery, mass lesions or infiltrative diseases, vascular or hypoxic injuries, and cerebral infections cause failure of the releasing and pituitary hormones—resulting in single or combined abnormalities. Cerebral edema or increased intracranial pressure is thought to restrict the blood flow to the hypothalamic pituitary area. Frequently encountered abnormalities are diabetes insipidus (DI), syndrome of inappropriate antidiuretic hormone (SIADH), and cerebral salt wasting. These syndromes cause abnormalities of sodium and water balance. Evaluation of volume status, urine and serum sodium, and osmolality are required to determine which syndrome is present. This evaluation is important because the treatment depends on which abnormality is present (Table 1).
Diabetes Insipidus
DI is caused by lack of vasopressin (antidiuretic hormone [ADH]), which causes water diuresis of more than 3 l/day, dehydration, and hypernatremia. The urine is dilute with urine osmolality of less than 300 mOsm/kg and urine specific gravity of less than 1.005. Urine osmolality greater than 800 mOsm/kg excludes DI. The diagnosis is usually made when dilute urine output exceeds 200 ml for 2 consecutive hours.2 A dramatic rise in serum sodium occurs in the ICU patient population unless fluids are aggressively replaced. In the neurosurgery patient population, the incidence of DI is 3.7% with a mortality of 70%.2 DI commonly occurs in association with severe brain injury and herniation. The treatment is fluid rehydration and vasopressin replacement via IV dDAVP 2 to 4 mcg/day or intranasally 10 to 60 mcg/day. Frequent monitoring of electrolytes and central venous pressure monitoring are necessary. The water deficit is calculated and slowly replaced. Caution should be used when severe hypernatremia is present, with half the water deficit replaced in 24 hours to avoid demyelination (Table 2).
Table 2 Formulas for Water Deficit and Sodium Deficit
SIADH and Cerebral Salt Wasting
The syndrome of inappropriate antidiuretic hormone and cerebral salt wasting are linked through a common cause—traumatic brain injury—and common result—hypotonic hyponatremia. Cerebral salt wasting is most often associated with subarachnoid hemorrhage, whereas SIADH is also associated with brain injury, tumors, and medications (Table 3).3 The cause of SIADH is excessive release of ADH that leads to water retention and an increase in extracellular fluid volume. Volume expansion increases renal sodium excretion, producing hyponatremia. Cerebral salt wasting is thought to involve disruption of the neural input to the kidney or central secretion of a natriuretic factor. Sodium is wasted by the kidney, causing extracellular volume depletion and stimulation of ADH secretion. Hyponatremia develops with extracellular volume depletion.
Table 3 Medications That Interfere with Thyroid Hormone and ADH
| Drugs Causing Syndrome of Inappropriate Antidiuretic Hormone (SIADH): |
Stimulating ADH release: opiates, opioids, barbiturates, nicotine, thiazides, isoproterenol, cyclic antidepressants, MAO inhibitors, haloperidol, risperidone, acetylcholine Stimulating ADH release and enhancing renal sensitivity: chlorpropamide, tolbutamide, cyclophosphamide, chlorambucil |
| Drugs That Influence Thyroid Function: |
Increase TSH: cimetidine, dopamine antagonists, haloperidol, iodide, lithium, contrast agents, metoclopramide |
NSAID, Nonsteroidal anti-inflammation drug.
In both syndromes, the diagnosis begins with serum sodium less than 135 mmol/l. Measurement of serum osmolality is less than 280 mOsm/kg, and the urine osmolality is greater than 100 mOsm/kg in both diseases. The differentiating factor is the effective blood volume, which is normal in SIADH and low in cerebral salt wasting. Low effective blood volume causes orthostatic blood pressure, tachycardia, low central venous pressure (CVP), low urine sodium, chloride, and fractional excretion of sodium with high BUN. Both disease states have low uric acid level. With correction of the salt deficit, uric acid levels normalize in SIADH and not in cerebral salt wasting.3 The treatment of SIADH is fluid restriction (800–1000 ml/day) and occasional hypertonic saline. Demelocycline, phenytoin, and lithium are used for chronic SIADH. The treatment for cerebral salt wasting is normal saline fluid replacement to expand the extracellular fluid compartment (see Table 1).
ABNORMALITIES IN THYROID RESPONSE
Untreated or unrecognized thyroid problems (excess or deficit) create life-threatening illness in critically ill patients. Thyroid hormones are responsible for the metabolic rate in all tissues. Critical illness may alter the production of thyroid hormone through thyroid-stimulating hormone (TSH) regulation, peripheral metabolism, or alteration in binding proteins. Cytokines as well as commonly used intensive care unit (ICU) medications (see Table 3) affect thyroid hormone function.
Thyroid Excess
Hyperthyroidism with a 3% incidence in out-patients is caused by Grave’s disease, goiter, and adenoma. Treatment with amiodarone increases the incidence to 9%.4 Thyroid storm (severe hyperthyroidism) was first recognized after thyroidectomy in unprepared patients and now encompasses 1%–2% of all admissions for thyrotoxicosis (with a mortality of 20%–30%).5 It is precipitated by physiologic stress related to specific events such as surgery, trauma, childbirth, severe illness, overdose of thyroid medication, iodine in medications, and contrast. The classic symptomatology includes fever, cardiovascular abnormalities, and mental status changes. The tachycardia is out of proportion to the fever. Fever is the hallmark of this disease, with temperatures to 106° F. A state of high-output cardiac failure can develop with bounding pulses, rales and hepatomegaly, and thyroidal bruit. Atrial fibrillation and congestive heart failure are common in elderly patients with hyperthyroidism and can occur without fever (thyrocardiac crisis). The mental status changes include a broad spectrum, from anxiety to coma. There can be nonspecific gastrointestinal (GI) complaints and clinical findings of Grave’s disease.
This syndrome is recognized by clinical signs and symptoms. Laboratory test turnaround time is long, and treatment should be started based on clinical suspicion. The TSH is not detectable, and both T3 and T4 are elevated (with T3 > T4).6 There is an associated elevation of white blood counts (WBCs), calcium, blood glucose, and liver function tests. The treatment is directed toward decreasing the production of thyroid hormone and preventing its release, blocking the peripheral action, supportive care, and treating the cause (Table 4).7
Table 4 Treatment of Thyroid Storm