Fig. 81.1
CT Scan abdomen, Grade 2 liver laceration with active contrast extravasation
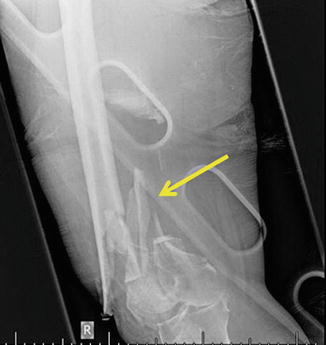
Fig. 81.2
Extremity radiograph with distal communited femur fracture
Postoperatively, she continued to have episodes of hypotension requiring continued resuscitation. Within the first 24 h, the patient received 6 L of crystalloid, 3U pRBC, and 500 cc of albumin. Despite the resuscitation, the patient developed acute renal failure requiring CVVH.
Question
What differential diagnoses should be considered?
Answer
Abdominal and extremity compartment syndrome.
Old age, trauma and high volume resuscitation are risk factors for abdominal and extremity compartment syndromes. Since clinical exam is unreliable in predicting intra-abdominal pressures (IAP), surveillance of intra-abdominal pressures using transbladder pressure monitoring should be implemented. The fractured leg should be assessed with serial exams and intracompartmental pressure measurements. For this patient, transbladder pressures were monitored every 4 h.
Postinjury day 3, she presented with poor oxygenation while still on mechanical ventilation with peak pressures of 41. IAP was found to be 29. She was taken emergently to the operating room for a decompressive laparotomy. Her open abdomen was managed using a damage control technique employing a negative pressure therapy dressing. She continued to experience hypotensive episodes requiring cystalloid boluses.
On post-decompression day 2, her nurse noted increased swelling of her right lower extremity. On physical exam, passive movement of the leg seemed to cause her “agitation” that would not improve with normal doses of pain medication. Intracompartmental pressures in her right anterior compartment showed an absolute value of 40 mmHg with a diastolic blood pressure of 57 mmHg  . An emergent 4 compartment fasciotomy was performed at the bedside. Muscle swelling without muscle necrosis was noted at the completion of the fasciotomy. Her fasciotomy wounds were initially managed with wet-to-dry gauze wraps with normal saline but were changed to a negative pressure therapy (NPT) dressing after 48 h. Her subsequent hospital course was notable only for a temporary abdominal coverage with a prosthetic mesh on postinjury day 10, followed by skin grafting on postinjury day 14. Delayed primary closure of her fasciotomy wound was achieved after 5 days of negative pressure therapy.
. An emergent 4 compartment fasciotomy was performed at the bedside. Muscle swelling without muscle necrosis was noted at the completion of the fasciotomy. Her fasciotomy wounds were initially managed with wet-to-dry gauze wraps with normal saline but were changed to a negative pressure therapy (NPT) dressing after 48 h. Her subsequent hospital course was notable only for a temporary abdominal coverage with a prosthetic mesh on postinjury day 10, followed by skin grafting on postinjury day 14. Delayed primary closure of her fasciotomy wound was achieved after 5 days of negative pressure therapy.
 . An emergent 4 compartment fasciotomy was performed at the bedside. Muscle swelling without muscle necrosis was noted at the completion of the fasciotomy. Her fasciotomy wounds were initially managed with wet-to-dry gauze wraps with normal saline but were changed to a negative pressure therapy (NPT) dressing after 48 h. Her subsequent hospital course was notable only for a temporary abdominal coverage with a prosthetic mesh on postinjury day 10, followed by skin grafting on postinjury day 14. Delayed primary closure of her fasciotomy wound was achieved after 5 days of negative pressure therapy.
. An emergent 4 compartment fasciotomy was performed at the bedside. Muscle swelling without muscle necrosis was noted at the completion of the fasciotomy. Her fasciotomy wounds were initially managed with wet-to-dry gauze wraps with normal saline but were changed to a negative pressure therapy (NPT) dressing after 48 h. Her subsequent hospital course was notable only for a temporary abdominal coverage with a prosthetic mesh on postinjury day 10, followed by skin grafting on postinjury day 14. Delayed primary closure of her fasciotomy wound was achieved after 5 days of negative pressure therapy.Principles of Management
Diagnosis
Compartment syndrome is a state of decreased tissue perfusion in a specific body compartment due to increased intracompartmental pressures from either interstitial edema, or increased intracompartmental contents or fluid. Early diagnosis is the key to successful management. Clinical exam alone has been shown to be inadequate for diagnosis of an acute compartment syndrome [1, 2]. Surveillance with a combination of serial clinical exams and intra-compartmental pressure monitoring is the most efficient approach to diagnosis in both the abdomen and extremity [3, 4].
Abdomen
Abdominal surgery, fluid resuscitation >3500 ml/24 h, ileus, pulmonary, renal, or liver dysfunction, hypothermia, acidosis, anemia, oliguria, and elevated GAP CO2 (gastric mucosal CO2 minus end-tidal CO2 tension) have been identified as risk factors for abdominal compartment syndrome in three prospective trials [5–7]. Patients with at least 2 risk factors should be surveyed with intra-abdominal pressure measurments taken via transbladder catheter. The measurements should be taken by instilling 25 cc of normal saline through the catheter and connecting the catheter to a pressure monitor. The patient should be supine and the monitor should be zeroed at the midaxillary line at the iliac crest. Patients sometimes require sedation and neuromuscular blockade to obtain an accurate intra-abdominal pressure reading via urinary catheter, as activity and abdominal muscle tensing will falsely elevate bladder pressures.
Organ dysfunction has been detected with IAPs as low as 10–15 mmHg [8]. The World Society of the Abdominal Compartment Syndrome (WSACS) has defined intra-abdominal hypertension (IAH) as a pathological state where the IAP is persistently greater than 12 mmHg. The spectrum of IAH is broken into 4 categories of increasing severity (Table 81.1).
Table 81.1
Intra-abdominal hypertension grading scale
Grade | IAP (mmHg) |
|---|---|
1 | 12–15 |
2 | 16–20 |
3 | 21–25 |
4 | 25 + |
Abdominal compartment syndrome is defined as an abdominal compartment pressure of greater than 20 mmHg (class 3 or greater) that is associated with new organ dysfunction [8]. Common organ dysfunction associated with abdominal compartment syndrome include respiratory (high peak and plateau airway pressures, hypercarbia and hypoxemia) and renal (oliguria, increasing serum creatinine, acute kidney injury) dysfunction.
Extremity
Risk factors for extremity compartment syndrome can be separated into fracture vs. non-fracture factors. Fracture-related risk factors include tibial diaphyseal fractures, soft tissue injury, crush injury and distal radial fractures [9]. Non-fracture risk factors include older age, greater number of comorbidities, presence of a coagulopathy such as hemophilia A or warfarin therapy, an increased base deficit, lactate and pRBC transfusion [10, 11]. Patients with at least two risk factors should undergo surveillance via serial clinical exams and intracompartmental pressure monitoring.
Clinical presentation of acute extremity compartment syndrome is most notably characterized by pain out of proportion to exam and pain with passive stretch [4]. Paresthesias and paralysis are late signs. These clinical signs have a sensitivity of 14–16 % but a specificity of 97 % [2]. Intracompartmental pressures can be obtained via commercial monitoring devices or an arterial blood pressure assembly. The threshold for diagnosing an acute compartment syndrome is a dynamic threshold termed 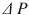 . It is defined as the difference between the diastolic blood pressure and the intracompartmental pressure. A threshold of less than 30 mmHg has generally been accepted as an indication for fasciotomy [12]. The sensitivity and specificity of continuous compartmental monitoring with a
. It is defined as the difference between the diastolic blood pressure and the intracompartmental pressure. A threshold of less than 30 mmHg has generally been accepted as an indication for fasciotomy [12]. The sensitivity and specificity of continuous compartmental monitoring with a 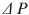 of less than 30 is 94 and 98 %, respectively [12].
of less than 30 is 94 and 98 %, respectively [12].
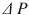 . It is defined as the difference between the diastolic blood pressure and the intracompartmental pressure. A threshold of less than 30 mmHg has generally been accepted as an indication for fasciotomy [12]. The sensitivity and specificity of continuous compartmental monitoring with a
. It is defined as the difference between the diastolic blood pressure and the intracompartmental pressure. A threshold of less than 30 mmHg has generally been accepted as an indication for fasciotomy [12]. The sensitivity and specificity of continuous compartmental monitoring with a 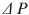 of less than 30 is 94 and 98 %, respectively [12].
of less than 30 is 94 and 98 %, respectively [12].Decompression
Definitive treatment of compartment syndrome involves surgical decompression of the compartment.
Abdomen
The gold standard for treatment of acute abdominal compartment syndrome is a decompressive laparotomy. Laparotomy is associated with a decrease in intra-abdominal pressure with improvement in cardiac, pulmonary and renal indices Despite these improvements, mortality still remains high at 46 % [13]. Temporizing measures have been developed to decrease intra-abdominal pressure in hopes of preventing an abdominal compartment syndrome such as sedation, supine positioning, and neuromuscular blockade [3]. Etiologies due to increased luminal or abdominal fluid collections could benefit from evacuation through nasogastric/orogastric suctioning and/or drainage of the fluid collections [3]. These measures should be implemented early before the development ofACS [3].
Extremity
Upon diagnosis of acute extremity compartment syndrome, emergent fasciotomy to release the compartment should be performed. The one exception is when there is suspicion that the compartment syndrome may have been ongoing for greater than 24 h. In a retrospective review of 336 combat veterans, delayed fasciotomy was associated with greater rates of muscle excision (25 vs. 11 %), amputation (31 vs. 15 %) and mortality (19 vs. 5 %) [14]. These results supported the findings of Finkelstein et al who described the clinical course in 5 patients who underwent delayed fasciotomy after 35 h of acute extremity compartment syndrome [15]. One patient died from multi-organ failure and septicemia, and the remaining four patients required amputations to treat refractory infections, multi-organ failure and sepsis. They speculated that after 24 h most of the tissues in the leg had died and by exposing that dead tissue to the open air, it provided a substrate for bacterial infection [15]. Thus, after 24 h, patients suffering from acute compartment syndrome, should probably be considered for amputation rather than fasciotomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







