Fig. 18.1
Initial chest x-ray with evidence of pulmonary edema and widened mediastinum
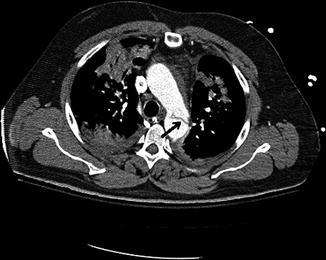
Fig. 18.2
CTA chest showing the dissection flap (black arrow) at the level of the descending aorta
Question
What is the initial approach to the management of a patient with acute aortic dissection?
Answer
Blood pressure control, evaluation for involvement of the aortic root, or end-organ malperfusion.
Acute aortic dissection, regardless of the location, requires strict blood pressure control and admission to an intensive care unit (ICU). Ascending aortic dissections are a surgical emergency and need immediate evaluation by cardiothoracic surgeons with emergent repair. All other dissections are usually managed with strict blood pressure control and urgent Cardiothoracic and Vascular Surgery consultations, unless there is evidence of end-organ hypoperfusion [1–3].
As pain can contribute to elevated blood pressures, the patient was given 4 mg Morphine IV with a modest improvement in his pain. However, he continued to have significant anxiety, shortness of breath and diaphoresis. After a discussion with the patient and his family, he was orotracheally intubated. An arterial line was placed into his right radial artery and his pressures were noted to be 170/100 s mmHg. Bilateral upper extremity cuff pressures correlated with pressures obtained from his arterial line. He was started on an esmolol drip at 10 mcg/kg/minute. This agent was quickly titrated up to 150 mcg/kg/minute (maximum infusion rate of 300 mcg/kg/minute) in order to obtain a goal heart rate <60 beats per minute (bpm) and a systolic blood pressure <120 mmHg. Despite high doses of esmolol with a heart rate <60 bpm, the patient’s blood pressure remained uncontrolled so a nitroprusside infusion was started at 0.25 mcg/kg/minute and increased up to 0.35 mcg/kg/minute (maximum dose 2 mcg/kg/minute). Blood pressures remained poorly controlled with the two agents and so a third agent, labetalol was added at 1 mg/minute and increased up to 6 mg/minute. The initiation of labetalol improved his BP and the esmolol drip was weaned off.
Concurrent to the patient’s blood pressure control, a STAT CTA chest was obtained upon the patient’s arrival, and was read as a dissection of the aortic arch starting at the innominate artery (brachiocephalic trunk) and extending to the descending aorta. Cardiothoracic Surgery and Vascular Surgery evaluated the patient and opted to medically manage him as the ascending aorta was not involved. Twelve hours later, a transthoracic echocardiogram (TTE) was performed and was concerning for aortic valve involvement. A transesophageal echocardiogram (TEE) was immediately performed which confirmed ascending aortic dissection with aortic insufficiency without pericardial tamponade (Figs. 18.3, 18.4, and 18.5, Video 18.1 and 18.2).
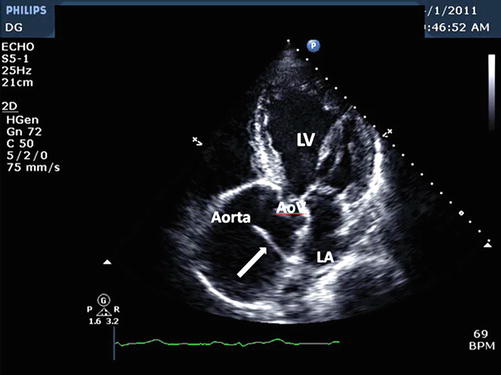
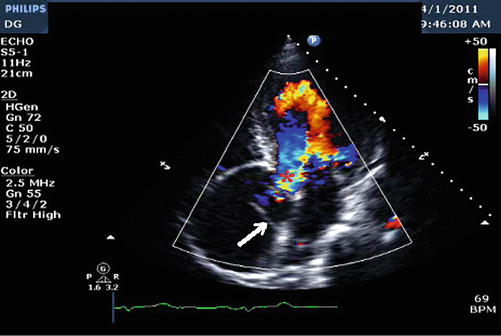
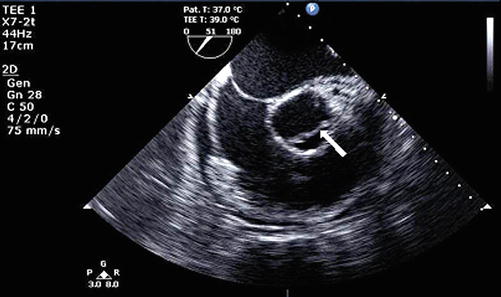

Fig. 18.3
Apical 5 chamber view demonstrating marked dilatation of the proximal ascending aorta and dissection flap (arrow). AoV aortic valve, LV left ventricle, LA left atrium (Image courtesy of Priscilla Peters, BA, RDCS, FASE)

Fig. 18.4
Apical 5 chamber view revealing aortic regurgitation. White arrow points to the dissection flap. Red asterisk (*) at area of aortic regurgitation (Image courtesy of Priscilla Peters, BA, RDCS, FASE)

Fig. 18.5
Off-axis transesophageal echocardiogram revealing an ascending aortic dissection with a dissection flap (arrow)
Principles of Management
Definition
Acute aortic syndromes comprise a spectrum of disease involving the aorta, including penetrating aortic ulcers (PAU), intramural hematomas (IMH), and aortic dissection [2, 4]. Intramural hematomas develop as a result of a micro-tear within the vasa vasorum resulting in an intramedial hematoma that is characterized by the absence of an aortic entry or exit tear. The prevalence of IMH in patients found to have aortic dissections ranges from 4 to 22 %. The IMH may evolve to overt dissection or even rupture, with progression that may occur suddenly or be heralded by ongoing acute aortic syndrome. Fifty to 80 % may resolve completely but patients may also progress to dissection or develop an aneurysm later in their disease course. Resolution occurs more often in younger patients, and those with aortic diameter < 4–4.5 cm, hematoma thickness <1 cm, and therapy with beta-blockers [1, 2, 5, 6].
Penetrating aortic ulcers represent atherosclerotic plaques that have ulcerated into the medial layer of the aortic wall. The high pressure pulsatile flow of blood through the aorta can cause further erosion of this ulcerated plaque which, in turn, can result in instability of the aortic wall and can lead to IMH, dissection, and even aortic rupture. These lesions are uncommon in the ascending aorta because high flow is protective against atherosclerosis. However, if present, they usually rupture and are commonly fatal. Lesions in the ascending aorta can initially be treated medically with close observation. PAUs more commonly arise in the descending thoracic aorta [2, 4–6].
Anatomically, the ascending aorta is the section proximal to the brachiocephalic trunk and the descending aorta is distal to the left subclavian artery. The DeBakey and the Stanford classifications are the two most frequently utilized classification systems. The DeBakey classification system divides the dissection into 3 types. Type I involves both the ascending and descending aorta; Type II affects the ascending aorta only; and Type III is a dissection distal to the left subclavian artery. The Stanford classification simplifies this further. The dissection either affects the ascending aorta (Type A) or it remains distal to the left subclavian (Type B) [7]. Isolated aortic arch dissections do not fit neatly into any of these classification types, and there is debate as to whether they should be grouped with Type A or Type B dissections. Some have argued that the natural history approximates more closely that of Type B dissections and as such, isolated arch dissections do not require emergent surgical intervention. Other experts disagree [7, 8] (Fig. 18.6).
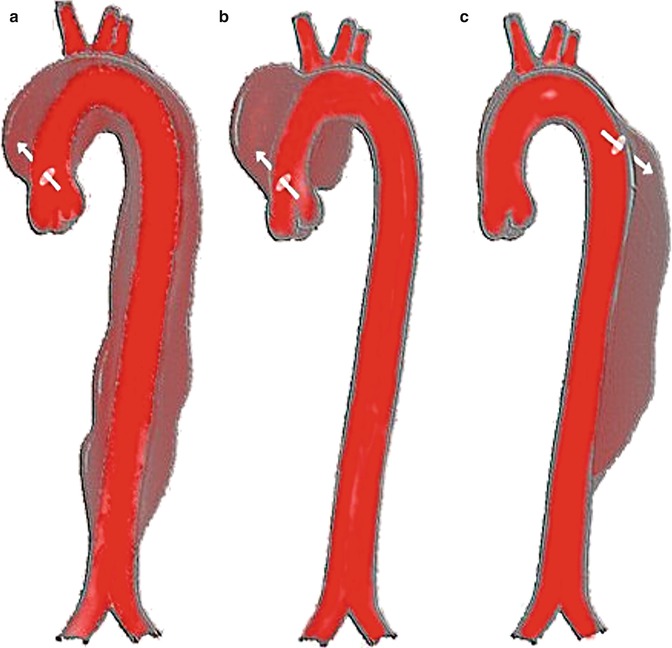

Fig. 18.6
Different types of aortic dissection. DeBakey classification: (a) DeBakey Type 1. (b) DeBakey Type 2. (c) DeBakey Type 3. Stanford classification: (a, b) Stanford Type A. (c) Stanford type B (Image courtesy of J. Heuser/Creative Commons)
Risk factors for acute aortic dissection are varied. Hypertension is a predominant risk factor [9] but other factors include bicuspid aortic valve, cocaine use, Marfan syndrome (and other connective tissue disorders), blunt trauma, pregnancy, weight lifting (Valsalva maneuvers), previous aortic surgery, and large-vessel vasculitis, among others [1].
Diagnosis
Chest pain is a ubiquitous presenting complaint. In a patient with hypertension and chest pain radiating to the back, acute aortic syndrome must be considered and ruled out. While angiography had previously been the gold standard, CTA of the chest is now the standard of care to diagnose aortic dissection given its widespread availability and ease of use. MRI may also be used to augment findings although it is not the diagnostic study of choice for emergently imaging the aorta due to lesser availability and longer duration of image acquisition. TTE and TEE are other modalities that may be used to evaluate the aorta, particularly in a hemodynamically unstable patient for whom transport out of the department would be unsafe [2, 10, 11]. Although the quality of echocardiograms is operator dependent, TTE generally has a sensitivity of 59–83 % and a specificity of 63–93 % for diagnosing an ascending aortic dissection [5]. TEE has a sensitivity of close to 100 % with a specificity of 89 % for identifying an ascending aortic dissection, although it can be as low as 31–55 % for descending aortic aneurysms [6].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







