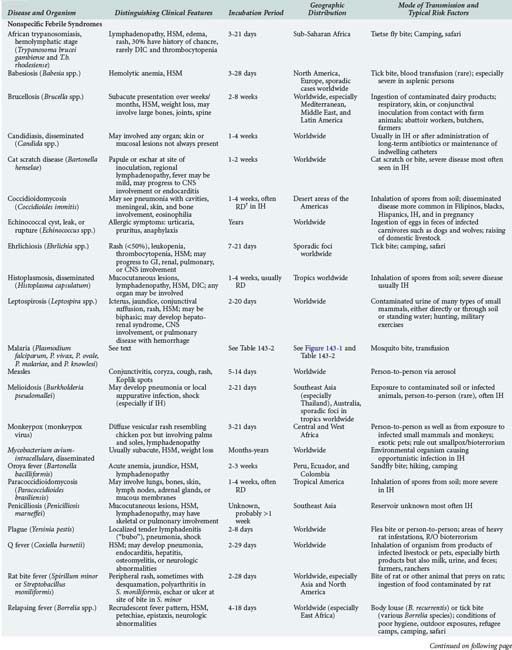
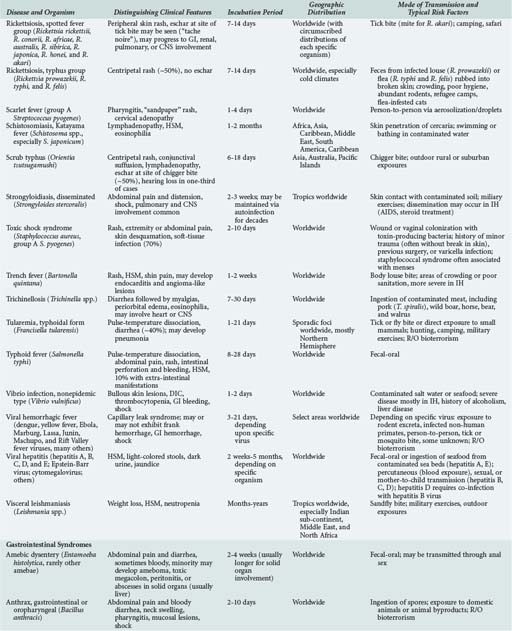
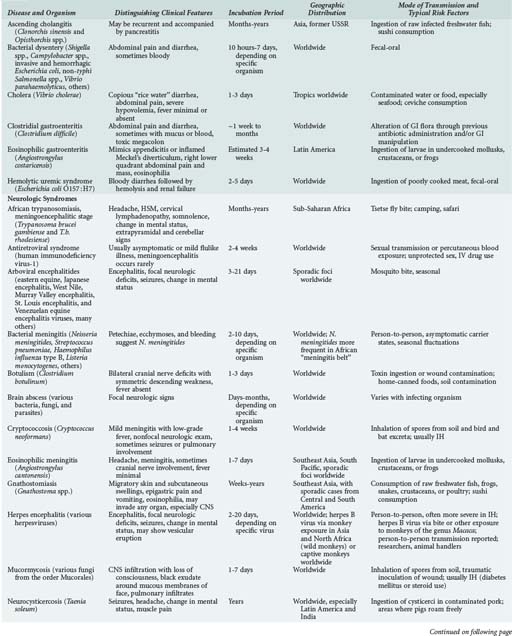
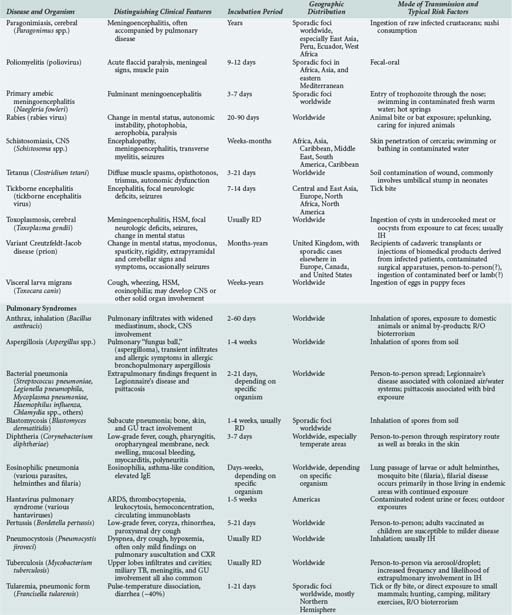
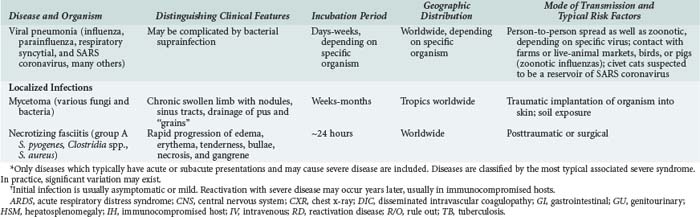
143 Malaria and Other Tropical Infections in the Intensive Care Unit
Although the spectrum of possible “tropical” infections in a patient with exposures overseas may initially seem daunting, a detailed history of the travel itinerary, activities, and exposures can often significantly narrow the differential diagnosis (Table 143-1). This must include more than simply recording the countries to which the patient traveled. Exposures of a business traveler staying at hotels and dining in fine restaurants in a major city may differ drastically from those of a student back-packing through rural areas of the same country. General knowledge of the diseases endemic in a given area and their incubation periods and drug resistance patterns is vital (Figure 143-1 and Table 143-2). In addition, most “non-tropical” infections are also common in developing countries. Thus, although the differential diagnosis must be expanded to include tropical pathogens, common illnesses seen in developing as well as industrialized countries must be considered.
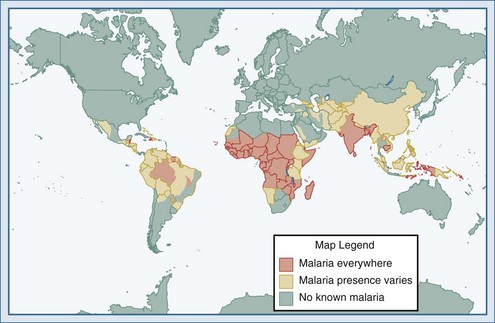
(From Health information for international travel 2010. Atlanta: Centers for Disease Control and Prevention; 2010. Available at: http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-2/malaria.aspx.)
Patients prone to tropical infections can be divided into three groups: (1) nonimmune persons who have no history of exposure to tropical pathogens, primarily tourists and young children, regardless of geographic origin, after the waning of maternal antibodies (around age 6 months); (2) immune or semi-immune persons residing in tropical countries who are repeatedly exposed; (3) those originally from tropical countries but now residing elsewhere who, in the absence of continued exposure, have waning immunity. The degree of immunity may exert profound effects on the presentation and severity of illness. For example, a returning traveler may develop severe malaria at a relatively low parasitemic load, whearas a resident of sub-Saharan Africa with the same degree of parasitemia may be asymptomatic. Genetic differences in susceptibility may also exist, such as resistance to Plasmodium vivax in blacks due to the absence of Duffy factor, which serves as the receptor, or the relative protection from severe malaria of any species afforded to those carrying the sickle cell trait.1,2,3
In returning travelers, knowledge of pre-travel vaccinations as well as prescribed and taken chemoprophylaxis (which often turn out not to be the same) is imperative. Nevertheless, these preventive measures do not confer 100% protection and should not be used to completely discard a given entity from the differential diagnosis. Both physicians and patients frequently err in the prescribing of and adherence to appropriate prophylactic regimens.4,5 Chemotherapy, complete or partial, may prolong the incubation period or alter the presentation of the illness. Those initially from tropical countries are often less likely to seek pre-travel medical advice before making a visit home and also often have considerably more exposures to tropical pathogens during their visit than do short-term travelers from industrialized countries.6
People living in resource-poor tropical countries may be more likely to have complicating health problems but less likely to have them previously diagnosed or controlled. Underlying diabetes, hypertension, malnutrition, chronic anemia, intestinal parasites, tuberculosis, HIV, or hepatitis virus infection may be discovered at the time of the acute illness.7 Infection with multiple tropical pathogens is common in those living in endemic areas. Thus the finding of a given pathogen cannot automatically be assumed to be the cause of the patient’s current illness.
 Epidemiology
Epidemiology
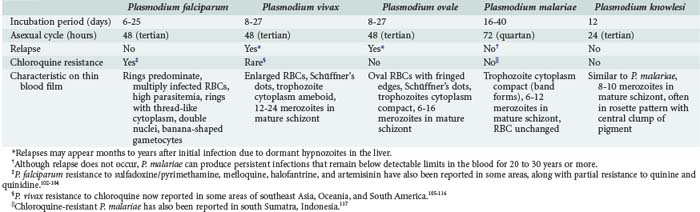
Malaria parasites are spread to humans by the bite of anopheline mosquitoes. Four species of Plasmodia commonly cause malaria in humans: Plasmodium falciparum, P. vivax, Plasmodium ovale, and Plasmodium malariae (see Table 143-2). A fifth species, Plasmodium knowlesi, is a zoonotic parasite of monkeys recently found to also cause disease in humans with exposure in forests of Southeast Asia.8,9 Furthermore, recent evidence suggests that there may be distinct species of P. vivax.10
Malaria is the most common serious infection in most tropical countries as well as in returning travelers, and it should therefore be considered in any patient reporting travel in malaria-endemic areas or with exposure to unscreened blood products (“transfusion malaria”) or blood-contaminated needles. Increased travel and immigration over the past several decades have resulted in increases in imported malaria in most industrialized countries.11,12 The risk of acquiring P. falciparum, the cause of most severe disease, is highest for those traveling to sub-Saharan Africa and New Guinea, moderate in India, and comparatively low in Southeast Asia and Latin America.13,14 Malaria is occasionally reported in individuals without reported travel, usually resulting from the carriage of malaria-infected passengers (who may be asymptomatic) or anopheline mosquitoes on aircraft arriving from endemic areas.15 The parasite may then be secondarily transmitted by anopheline mosquitoes endemic in some industrialized countries, including the United States.
 Pathophysiology
Pathophysiology
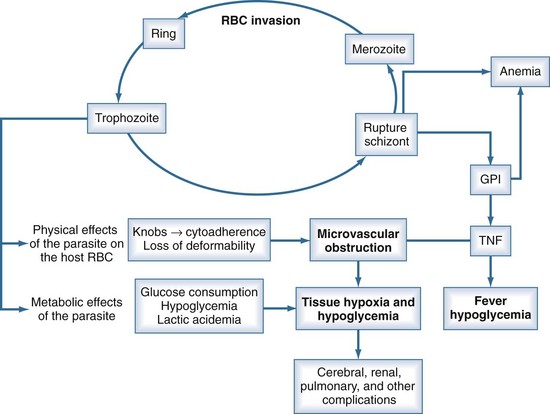
P. falciparum accounts for the vast majority of severe malaria because of (1) its ability to infect red blood cells (RBCs) of all ages, resulting in overwhelming parasitemia (up to 70% of RBCs); (2) its induction of adherence of parasitized RBCs to the microvascular wall, with consequent obstruction; (3) its induction of severe metabolic derangements directly through glucose consumption and lactate production and indirectly through the induction of cytokines; and (4) the high prevalence of chloroquine resistance to P. falciparum in many parts of the world (see Table 143-2). Nonimmune persons and pregnant women are at greatest risk. Human genetic as well as parasite strain differences probably play roles in the ultimate course of any given malaria infection.
Unlike the other species of malaria, P. falciparum causes decreased RBC deformability and the production of small protrusions or “knobs” on parasitized RBC membranes that mediate their adhesion to the venular endothelium (Figure 143-2). The rupture of schizont-stage parasites exposes glycosylphosphatidylinositol anchors on the parasite and RBC surface that induce macrophages and other inflammatory cells to release a host of inflammatory mediators including tumor necrosis factor alpha (TNF-α), interleukin-1, TNF-β, and various kinins and reactive nitrogen intermediates.16–18 These cytokines play a role in up-regulation and activation of endothelial adhesion molecules such as ICAM-1 and E-selectin, enhancing cytoadherence of parasitized cells as well as mediating pathologic processes such as hypoglycemia, lactic acidemia, shock, gut mucosal damage, and increased permeability and neutrophil aggregation in the lung. The sum total of this cascade is sequestration of parasitized RBCs in the microvasculature where they are not only sheltered from removal but cause sluggish flow and obstruction, resulting in impaired oxygen delivery and organ dysfunction.16,19 The most profound effects are usually on the cerebral capillaries, although a host of tissues may be affected, including the kidney, liver, spleen, placenta, intestine, lung, bone marrow, heart, and retina. Histopathologic changes are usually minimal, but ring hemorrhages and perivascular infiltrates sometimes develop at the sites of obstructed vessels, perhaps facilitated by thrombocytopenia due to splenic sequestration of platelets. Although subendocardial and epicardial hemorrhages have been noted at autopsy, myocarditis does not occur, and primary cardiac events are relatively rare in malaria.
 Clinical Presentation
Clinical Presentation
Although a classic periodicity is described for the different malaria species (see Table 143-2), this occurs only when the infection has persisted untreated long enough to allow for synchronization of schizont rupture. Furthermore, schizont rupture tends to be asynchronous in P. falciparum and in most primary infections of any plasmodium species. Therefore, malaria may often result in persistently spiking fevers difficult to distinguish from fever produced by many other infections. The absence of a classic paroxysm and periodicity therefore should not be used to exclude the diagnosis. Paroxysms may be accompanied by cough, sore throat, myalgias, back pain, postural hypotension, abdominal pain, nausea, vomiting, diarrhea, and weakness. These are more common in children and may lead to misdiagnoses. Rash and lymphadenopathy are not typical of malaria and suggest another diagnosis.
Severe and Complicated Malaria
Although all species of malaria may produce severe consequences in a debilitated patient, potentially fatal malaria which merits attention in an ICU can be grouped into three categories: (1) severe complications of P. falciparum in nonimmune children and adults, responsible for the vast majority of severe disease worldwide (Table 143-3); (2) splenic rupture, which occurs most frequently with P. vivax; and (3) chronic nephrotic syndrome due to immune-complex nephritis associated with P. malariae, usually seen in children and often complicated by overwhelming bacterial infection. There is emerging evidence that P. knowlesi can also cause severe fatal malaria and should be treated in an ICU setting.8
TABLE 143-3 Clinical and Laboratory Features That Classify a Patient as Suffering from Severe Plasmodium falciparum Malaria According to the World Health Organization
Modified from Guidelines for the treatment of malaria 2010. Geneva: World Health Organization; 2010. Available at: http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf.
Cerebral Malaria
This is the most frequent severe complication of plasmodium infection, accounting for most fatalities as well as chronic sequelae. It is most frequent in children of 3 to 5 years of age. Strictly defined, cerebral malaria implies unarousable coma due to P. falciparum.20,21 Hyperpyrexia and febrile convulsions in young children may produce transiently altered mental status without true involvement of the cerebral microvasculature and thus technically do not constitute cerebral malaria. However, in clinical practice, seizures or persistent changes in sensorium which cannot be attributed to other disease processes should be considered cerebral malaria until proven otherwise. Although cerebral malaria is classically attributed to cytoadhesion and microvascular obstruction in the brain, other ongoing processes including hypoglycemia, metabolic acidosis, and impaired oxygenation due to anemia and pulmonary edema likely contribute.
The altered sensorium of cerebral malaria may develop gradually within a few days of onset of illness or manifest as persistent coma after a generalized convulsion. Compared to adults, children with cerebral malaria have a shorter history of fever before progressing to coma (average about 2 days). The most common neurologic picture is of a symmetrical upper motor neuron lesion with hypertonia, hyperreflexia, clonus, absent abdominal reflexes, and extensor Babinski responses. Hypotonia and acute cerebellar ataxia are sometimes seen as well, especially in India and Sri Lanka. There is usually a diffuse symmetric encephalopathy, sometimes with signs of frontal lobe release such as a pout reflex or bruxism. There is usually no grasp reflex, and the gag reflex is normally maintained. Both decorticate as well as decerebrate posturing may occur.21 Meningismus, opisthotonos, and disconjugate gaze are frequently seen. Nystagmus and a sixth nerve palsy are rare. Pupils are usually symmetric with intact pupillary, corneal, oculocephalic, and oculovestibular reflexes. Photophobia, severe neck rigidity, and papilledema are almost never seen.
Convulsions may occur in up to 50% of cases of cerebral malaria. As a child ages above 3 to 4 years, seizures become more likely to represent cerebral malaria rather than febrile convulsions.22 Although generalized seizures are classically reported, partial motor seizures, with or without secondary generalization, may occur. 20 Although often showing only diffuse cortical dysfunction, EEG studies may sometimes reveal underlying status epilepticus even when it is not clinically evident.21
Pulmonary Edema and Acute Respiratory Distress Syndrome
Pulmonary edema, which may progress to acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), is frequent and typically the most lethal of the complications of malaria. Recent evidence suggests that the mechanism may involve acute pulmonary hypertension precipitated by nitric oxide consumption by free plasma hemoglobin released from intravascular hemolysis.[Janka et al., in press] Endothelial injury leading to increased alveolar permeability and noncardiogenic pulmonary edema may also contribute. Interstitial edema and inflammatory cell infiltrates are seen at autopsy, but sequestration of parasitized RBCs in the lung is uncommon.23 Pulmonary complications occur in 5% to 30% of patients with severe malaria, especially pregnant women, nonimmune persons, and patients already suffering from other complications.23 The onset may be any time during the course of illness, even if the patient appears to be improving and parasitemia has decreased. Symptoms include dyspnea and cough, with rapid progression to hypoxia and respiratory distress.
Anemia and Hematologic Perturbations
Although some degree of anemia is common in all types of malaria, severe anemia (hemoglobin less than 5 g/100 mL) occurs almost exclusively with P. falciparum infections, owing to their high parasitemias. It is most common and often severe in pregnant women and young children (<1 year), in whom it may be the presenting sign.24 In addition to the acute hemolytic destruction of parasitized RBCs, the more chronic processes of removal of parasitized cells from circulation by the spleen and cytokine inhibition of erythropoiesis may contribute.25 Nonimmune subjects may develop anemia within days after infection, whereas anemia usually develops more slowly in those who are semi-immune. The degree of anemia generally correlates with bilirubin level and level of parasitemia. It may be exacerbated by underlying glucose-6-phosphate dehydrogenase (G6PD) deficiency in the setting of administration of oxidant antimalarial drugs (e.g., quinine, sulfadoxine) and iron-deficiency anemia due to malnutrition. Significant jaundice and hemoglobinuria may result. Thrombocytopenia, although frequent, is not usually associated with bleeding or correlated with disease severity. Disseminated intravascular coagulation (DIC) is seen in less than 10% of severe cases.
Acute Renal Failure
Blackwater fever refers to a severe syndrome characterized by low or absent parasitemia, intravascular hemolysis, hemoglobinuria, and ARF. It is classically seen in people of northern European descent chronically exposed to P. falciparum and irregularly taking the quinoline antimalarial drugs, quinine or quinidine, which together are known as the cinchona alkaloids. The syndrome virtually disappeared after 1950 when chloroquine superseded quinine. However, it is now said to be resurgent, albeit with lower mortality, in relation to mounting chloroquine resistance and consequent increased use of quinine and the newer quinolines, such as mefloquine and halofantrine.26
Shock and Bacterial and Other Suprainfection
So-called algid malaria, referring to hypotension and shock, may resemble and indeed sometimes be due to gram-negative sepsis from impaired flow in intestinal capillaries, with resultant mucosal erosion. Non-typhoidal salmonella septicemia is specifically associated with P. falciparum.27 Algid malaria is often seen in the setting of hyperparasitemia, with concomitant hypoglycemia and lactic acidemia, and may progress to multiorgan system failure and death. As with most malaria complications, severe hemodynamic derangements are most often seen in nonimmune persons.28 Whether bacteria are isolated or not, a classic septic shock picture is typical, with elevated cardiac index and decreased systemic vascular resistance.29 Hemodynamic decompensation due to splenic rupture may mimic algid malaria.
A host of other infectious complications, including aspiration pneumonia and parvovirus infection, may be related to falciparum malaria. Malaria occurs with increasing frequency and severity in those who are human immunodeficiency virus (HIV) infected, especially during pregnancy, and can also transiently up-regulate HIV replication.30–34 An association between severe malaria infection and hepatitis B surface antigen carriage has also been noted.35
Tropical Splenomegaly and Splenic Rupture
Splenomegaly is common in infection with all species of malaria. The tropical splenomegaly syndrome, also sometimes termed hyperreactive malarial syndrome (HMS), refers to a condition of massive splenomegaly, high titers of total serum IgM and malaria-specific antibodies, and scanty or absent parasitemia. It is seen in individuals with a history of residence in an endemic area and can be associated with any malaria species. Host genetic factors appear to play a role.36
Unlike virtually all the other complications of malaria that are most often associated with P. falciparum, acute splenic complications occur most commonly in P. vivax, especially with the first infection. Although the term spontaneous splenic rupture has traditionally been used, in reality a range of hematomas or tears of varying severity may occur. The rupture or tear usually occurs 2 to 3 months after infection, presumably due to increased intrasplenic tension, often precipitated by trauma of varying degrees or mechanical ventilation.37 Over-eager examiners have been suggested to play a role, although no cases of clear palpation-induced rupture have been reported. Fever, tachycardia, vomiting, prostration, abdominal pain or guarding, tender splenomegaly, hypovolemia, and rapidly worsening anemia are common presenting features. Abdominal pain may be localized or diffuse, mild or severe. Shock may ensue. Diaphragmatic irritation after rupture may cause referred pain to the left shoulder, supraclavicular, or scapular regions (“Kehr’s sign”). This is present in about one-half of cases and is said to have good specificity for rupture.
Malaria in Pregnancy and Children
In addition to being more susceptible to infection, malaria is particularly dangerous in pregnant women and their fetuses, with increased risk of pulmonary edema, hypoglycemia, severe anemia, premature delivery, low birth weight, and maternal and fetal death. Malaria parasites can often be found in the placenta and may impair oxygen and nutrient transport to the fetus. Disease is most severe in primiparae, especially if nonimmune. In contrast, women from endemic areas are usually asymptomatic, with the exception of the effects of anemia, again more severe in primiparae. Congenital malaria is rare except in those infants born to nonimmune mothers.38
 Diagnosis
Diagnosis
Clinical
Malaria often presents with nonspecific signs and symptoms, so making a clinical diagnosis may be difficult. Although almost all patients have a history of fever, they may frequently be afebrile at the time of examination.39 Physicians in industrialized countries who are unfamiliar with the disease may not initially include malaria in the differential diagnosis. Delayed diagnosis is frequent and associated with a poor outcome.6,40 Although patients with other species of malaria parasite may not present for months or even years after infection, the vast majority of those with P. falciparum will present within 6 months of exposure.4 The differential diagnosis includes most febrile illnesses found in the tropics (see Table 143-1). Babesiosis may present both clinically and microscopically similar to malaria in patients without travel to malaria-endemic areas. Cerebral malaria must be distinguished from bacterial meningitis, the viral meningoencephalitides, metabolic coma, and intoxications by lumbar puncture.41 In cerebral malaria, the cerebrospinal fluid (CSF) opening pressure is usually normal, although a few lymphocytes and moderate elevation of protein may be seen. High CSF lactate and low glucose indicate a poor prognosis.
Conventional Microscopy
Laboratory diagnosis has traditionally been made via the examination of thick and thin Giemsa-stained smears. Thick smears are more sensitive in diagnosing malaria, whereas thin smears allow identification of the specific parasite. Either smear can be used to quantify the level of parasitemia, but thick smears are theoretically more sensitive for this purpose.42,43 Simultaneous infections with multiple strains of P. falciparum are common in some areas of sub-Saharan Africa and also may occur with P. vivax in Southeast Asia and Latin America.44,45 Blood obtained by pricking a fingertip or earlobe is preferred because parasite densities are higher in these capillary-rich areas, although blood obtained by venipuncture collected in heparin or EDTA anticoagulant-coated tubes is acceptable if used shortly after being drawn (to prevent alteration in the morphology of white blood cells and malaria parasites).46 Smears should be taken as soon as the diagnosis of malaria is considered, without waiting for manifestation of a classic paroxysm. Parasitemia may be undetectable in the early stages of the illness, in those with partial immunity, and in those who have previously self-administered antimalarials, a common practice in malaria-endemic areas.47 Levels of parasitemia may fluctuate over time, necessitating repeated smears for diagnosis. Furthermore, P. falciparum–parasitized red blood cells may be sequestered in the deep capillaries of the spleen, liver, and bone marrow. Although a blood film is unlikely to be falsely negative in a patient with severe disease, negative smears should not prevent prompt administration of antimalarial therapy if the diagnosis is strongly suspected.14 Conversely, asymptomatic parasitemia is common in children from endemic areas, and thus a positive smear does not necessarily signify a clinical case under these circumstances.
Considerable expertise at reading malaria smears may be necessary to detect and distinguish the parasites (see Table 143-2). The most important point is to distinguish P. falciparum, with its concomitant risk of severe complications, from the other plasmodia. Superimposed platelets, particles of stain, pits in the slide, RBC inclusions such as Howell-Jolly bodies and those seen in siderocytes, and other intracellular pathogens such as Bartonella and Babesia must be distinguished from malaria parasites. Furthermore, alterations in parasite morphology may occur related to strain variation, drug pressure, and blood collection method.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree