Fig. 29.1
Initial assessment of lower gastrointestinal bleeding
An important diagnostic caveat must be kept in mind in the evaluation of lower gastrointestinal bleeding, specifically that multiple sources of bleeding are not infrequently identified in this patient population. Among patients admitted in the acute care setting for lower gastrointestinal bleeding, the number of patients with multiple sources of hemorrhage is estimated at 4.4% [13]. In a prospective study of patients presenting with a chief complaint of intermittent bright red blood per rectum, Graham and colleagues documented additional abnormal findings on colonoscopy in 27% of patients with identifiable abnormalities on rectal examination [15]. The workup is therefore not complete once a single likely source of bleeding is identified; rather, optimal patient care dictates that a comprehensive evaluation be completed and other reasonably likely etiologies ruled out clinically.
As with any clinical situation, a thorough evaluation must begin with a detailed history and physical examination. A relevant history for the evaluation of lower gastrointestinal bleeding should, at a minimum, address the following areas:
Acute bleeding symptoms: What is the nature of the bleeding? Is the patient experiencing hematochezia or melena? While traditional clinical dogma holds that hematochezia signifies a lower gastrointestinal bleed while melena is indicative of an upper gastrointestinal source of hemorrhage, the clinical reality is frequently less clear-cut, and it is widely acknowledged that particularly brisk upper gastrointestinal bleeding can present with hematochezia. Is the bleeding continuous or intermittent? Lower gastrointestinal bleeds are, in fact, intermittent in nature, making localization a true diagnostic challenge. How long has the bleeding been occurring? Has the patient experienced previous episodes of upper or lower gastrointestinal bleeding? Is there any pain associated with the bleeding?
Possibly related systemic symptoms: Is the patient experiencing angina, palpitations, syncope, of unusual fatigue? Does the patient report any fevers or chills? Is nausea or vomiting present? Is there associated diarrhea or constipation? Does the patient report a history of gastroesophageal reflux or antacid use? Has there been any recent unintentional weight loss?
Relevant medical history: Has the patient previously experienced any type of upper or lower gastrointestinal bleeding? Any history of inflammatory bowel disease, diverticulosis, hemorrhoids, gastrointestinal neoplasm, liver disease? Does the patient report any history of gastric or duodenal ulcer? Is there a known history of atrial fibrillation or other cardiac dysrhythmia? Does the patient report a history of peripheral vascular disease or ischemia? Any history of hematologic disorders, including thrombocytopenia or clotting cascade abnormalities? Has the patient ever experienced a transient ischemia attack or cerebrovascular accident? Has the patient recently been treated with radiation therapy?
Medication history, including both prescription and nonprescription agents as well as herbal preparations. Specific inquiry regarding warfarin, aspirin, nonsteroidal anti-inflammatory agents, or other anticoagulant agents is of obvious importance.
Health maintenance: Has the patient undergone any health screening that might reveal gastrointestinal disease, such as fecal occult blood testing, flexible sigmoidoscopy, or colonoscopy? When were these studies done, and what were the results? Has the patient recently had a polypectomy performed?
Family history: Any relatives with any form of cancer, particularly cancers of the gastrointestinal tract? Any relatives with a history of inflammatory bowel disease? Any record of hereditary coagulopathies or other hematologic abnormalities?
Social history: Is there any history of alcohol and/or tobacco usage? Recent travel, particularly to less-developed countries or regions? Recent sick contacts?
A focused yet thorough physical examination is also indicated as a key element of the initial workup. Vital signs will often be within normal limits in the setting of a lower gastrointestinal bleed unless the rate of bleeding is so substantial as to cause a significant volume depletion effect; in that case, tachycardia would be observed somewhat earlier, while hypotension and/or altered mental status would represent later findings associated with the acute loss of greater than 30% of the circulating blood volume (class III or higher hemorrhagic shock) [16]. Any evidence of vital sign alteration due to blood loss should prompt immediate placement of large-bore peripheral access and the institution of aggressive resuscitation with crystalloid and/or, in especially severe cases, blood products. In this circumstance, restoration and stabilization of volume status is the clinician’s priority, and the further detailed physical examination is accordingly deferred until physiologic stability has been achieved.
A generalized visual inspection of the patient should reveal any anemic pallor, jaundice, or cachexia which might be present and associated with particular underlying conditions that could be associated with lower gastrointestinal bleeding. The abdominal examination should evaluate for generalized or focal tenderness, firmness or rigidity, any peritoneal signs such as guarding or rebound, organomegaly, and the presence of palpable masses. Presence of pain on abdominal examination generally argues in favor of an inflammatory process, while lower gastrointestinal bleeding due to diverticular disease or angiodysplasia in more commonly associated with a benign abdominal examination. Importantly, in the setting of a lower gastrointestinal bleed of unclear etiology, the examining clinician should perform a cardiac and peripheral pulse examination with particular attention to evidence of atrial fibrillation.
The rectal examination is among the most critical components of the physical examination in the patient with an acute lower gastrointestinal bleeding. A thorough and complete rectal exam should establish the presence or absence of gross blood, the existence of internal or external hemorrhoids or other perianal lesions including fistulae or fissures, and the presence and position of any palpable rectal masses. If no gross blood is apparent upon rectal examination, a stool guaiac test can be quickly performed in either the clinic or emergency department setting to establish the presence of occult gastrointestinal bleeding. Be aware, however, that the sensitivity of this assay is relatively low [17], and is further reduced in patients who take iron supplements or who have recently consumed red meat or peroxidase-rich fruits and vegetables, and specificity is reportedly diminished if a patient’s diet is rich in citrus fruits or other concentrated sources of vitamin C [18, 19].
Initial laboratory studies should be sent to aid in the immediate evaluation of both the etiology and magnitude of a lower gastrointestinal bleed. A complete blood count (CBC) might be expected to reveal a decreased hematocrit in a patient with an active gastrointestinal hemorrhage; however, if the hemorrhage is of particularly acute onset, the intravascular volume may not yet be fully re-equilibrated and thus the hematocrit may be artificially elevated relative to true oxygen-carrying capacity. The CBC would also be expected to reveal evidence of thrombocytopenia, albeit with the same caveat that a hyperacute process might not permit an adequate intravascular re-equilibration interval before the laboratory study is drawn. Presence of a significant leukocytosis on CBC should prompt further consideration of an infectious process as the inciting etiology versus an inflammatory or ischemic mechanism.
Basic laboratory studies of electrolyte status as well as hepatic and renal function may serve the dual purposes of elucidating underlying comorbidities which may contribute to a gastrointestinal bleed while also identifying physiologic imbalances which could potentially be corrected prior to surgical or other procedural interventions. Likewise, coagulation parameters in this patient population may uncover underlying coagulopathies contributing to the presenting problem and permit the practitioner to order blood products where appropriate. It should be noted that the routine administration of vitamin K to correct patients on chronic warfarin therapy should be avoided in the setting of a lower gastrointestinal hemorrhage due to the difficulty and delay this presents when attempting to reestablish therapeutic anticoagulation once the acute hemorrhagic episode has been resolved [8].
Radiographic imaging may play an important role in establishing a definitive diagnosis in patients with lower gastrointestinal bleeding. Most patients with lower gastrointestinal bleeding who report concurrent abdominal pain acutely will undergo plain abdominal radiographs prior to the surgical consult. The information gleaned from these studies is somewhat limited; however, findings such as pneumoperitoneum or closed-loop obstruction may narrow the differential diagnosis That being said, the utility of plain abdominal radiographs is of limited utility in the evaluation of lower gastrointestinal bleeding.
Most patients in the acute setting of lower gastrointestinal bleeding with concurrent abdominal pain will, if hemodynamically stable, be appropriate candidates for computed tomography (CT) scanning of the abdomen and pelvis. A CT with oral and intravenous contrast may help identify mass lesions, such as colorectal adenocarcinomas, as well as sites of inflammation or potential perforation, as is seen with acute diverticulitis or inflammatory bowel disease. Bowel wall thickening or pneumatosis may also be noted in the case of ischemia or hypoperfusion-mediated bowel injury; an acute thromboembolic process would be expected to demonstrate these types of pathologic changes within a discrete vascular territory, while a more global low-flow mechanism would be expected to generate corresponding diffuse bowel involvement. Optimally, a CT scan in this setting would be performed with the administration of both oral and intravenous contrast. The patient’s history should be reviewed for mention of impaired renal function or radiographic contrast allergy; initial laboratory studies including blood urea nitrogen and serum creatinine should likewise be reviewed prior to contrast administration.
Ultimately, the majority of patients undergoing an evaluation for lower gastrointestinal bleeding will undergo a colonoscopy. In addition to its utility as a diagnostic study, colonoscopic evaluation offers the advantage of potential therapeutic interventions. In acute lower gastrointestinal bleeding, the reported diagnostic utility of colonoscopy ranges between 45 and 89% [7, 20–23]. Complications of colonoscopy in the acute care setting, most significantly perforation, occur in up to 3% of cases [24].
The utility of colonoscopy in the acute setting is influenced by a number of factors including the quality of bowel preparation prior to the procedure, the rate of active bleeding (very slow bleeds may be below the diagnostic threshold of the procedure, while very brisk bleeding may impair adequate visualization and source localization), whether or not the bleeding is continuous or intermittent, and the skill/experience of the endoscopist. Additionally, not all facilities have 24-h availability of this procedure.
The quality of bowel preparation that can be achieved prior to colonoscopy has a clear influence on the success of the procedure from both a diagnostic and therapeutic perspective. That being said, a lack of bowel preparation does not preclude the successful use of endoscopic techniques in the diagnosis and treatment of lower gastrointestinal bleeding. In fact, some clinicians report that lower gastrointestinal bleeding actually acts to help purge the colon, and any impaired visualization on colonoscopy can be addressed via flushing the scope during the procedure, although diagnostic yield in this circumstance is only about 35% [23]. If a routine oral electrolyte-polyethylene glycol prep solution is administered prior to colonoscopy in the setting of an acute lower gastrointestinal bleed, improved diagnostic yields, approaching 80% are reported [25].
If colonoscopy is performed for acute lower gastrointestinal bleeding, and a definitive source is identified, therapeutic options include the following: sclerotherapy via direct epinephrine injection in a 1:10,000 concentration, bipolar or monopolar coagulation, and endoscopic clip application. Jensen and colleagues directly compared urgent colonoscopic intervention versus surgical treatment in a prospective study of patients with severe diverticular bleeding and demonstrated comparable efficacy [26].
In cases where resource issues or other patient factors make colonoscopy an unsuitable clinical option, flexible sigmoidoscopy may be utilized for visualization of the distal gastrointestinal tract. In cases in which a hemorrhagic lesion is identified within this segment of the colon, sigmoidoscopy can prove to be a valuable clinical adjunct for both diagnostic and treatment purposes. One must keep in mind that a significant portion of patients with distal lesions are also found to have more proximal sources of hemorrhage [15]; therefore, the performance of flexible sigmoidoscopy does not obviate the requirement for a more thorough examination via a complete colonoscopy at a later point in time.
If an anorectal source of bleeding is evident on examination or is suspected based on the clinical history and patient presentation, anoscopy is another tool which may be utilized to facilitate direct visualization and examination. Again, the identification of a distal lesion as a source of lower gastrointestinal hemorrhage does not in any way preclude the existence of a more proximal lesion. Therefore, it is advisable that these patients also be scheduled for a complete colonoscopy at a later date.
While colonoscopy is the preferred initial investigation for lower gastrointestinal bleeding [7, 27], angiography is another modality that offers the advantage of both diagnostic and therapeutic capabilities if colonoscopy is unavailable. The sensitivity for visceral angiography in the detection of active gastrointestinal bleeding is approximately 0.5 cm3/min [10, 28]. Angiography is similarly poor in detecting venous bleeding, intermittent bleeding, and bleeding from small vessels. Finally, angiography is not without complications to include: hemorrhage at the catheter insertion site, arterial dissection, microembolization, pseudoaneurysm formation, puncture site infection, allergic reaction to contrast, and contrast-induced nephropathy [20, 29].
The reported success rates for angiography in the localization of lower gastrointestinal bleeding vary widely, with recent studies citing rates between 30.5 and 86% [7, 12, 30]. If angiography is able to detect a discrete bleeding source, several therapeutic interventions are possible including: embolization therapy and direct injection of vasopressin or sclerosing agents at the bleeding site. Unfortunately, angiographic capabilities are not available on a 24-h basis universally. If a significant delay in angiography is anticipated, other diagnostic and therapeutic modalities should be considered.
Radionuclide scintigraphy is yet another diagnostic modality for identification of the site of hemorrhage in a patient presenting with lower gastrointestinal bleeding. This technique can utilize either technetium-99m sulfur colloid or technetium-99m-labeled red blood cells. The latter technique, commonly referred to as a tagged red blood cell scan, is utilized more frequently. Sulfur colloid scanning has the advantage of relative ease of preparation in comparison with preparation of tagged red blood cells. However, it clears quickly, thus decreasing the likelihood of repeat scanning following a single infusion (an option with tagged red blood cell scans). All that being said, the detection rates are similar between the two techniques [31].
Radionuclide scintigraphy is able to identify bleeding at rates as low as 0.1 cm3/min [32]. Thus, the tagged red blood cell scan is of greatest utility in identifying slow bleeds that are not localizable via other diagnostic techniques. Ng and colleagues evaluated the question of whether time to positive radionuclide scan (“blush”) correlates with, and can be used to predict, the yield on angiographic intervention. In their series, 60% of patients with an immediate appearance of blush on radionuclide scan subsequently underwent a positive angiogram. Among patients in whom no blush had appeared after 2 min, only 7% had a positive angiogram [33]. While sensitivity of the tagged red blood cell scan can surpass either colonoscopy or angiography in the setting of active bleeding and can be used to predict which patients will benefit from angiogram, radionuclide scanning does have the significant disadvantage of representing a diagnostic modality only, with no capability for direct therapeutic intervention. Furthermore, 27% of patients who undergo a negative radionuclide study will experience recurrent lower gastrointestinal bleeding at a later date [34].
Despite these many modalities, bleeding will cease spontaneously and no definitive source of lower gastrointestinal bleeding will occur in 10.7–22.8% of patients [2, 22, 35–37]. However, it must be emphasized that a thorough workup which fails to identify a definitive source of bleeding is not without benefit to the patient, in that a number of potentially serious causes of lower gastrointestinal bleeding, such as colorectal adenocarcinoma, can be effectively eliminated from the differential diagnosis following the workup.
Management
In the majority of cases (70–85%), the lower gastrointestinal bleeding will cease without any therapeutic intervention (Table 29.1) [8, 9]. Re-bleeding is not uncommon, occurring in up to 25% of cases [38]. Thus, the absence of active bleeding at a particular point in time should not preclude definitive evaluation and treatment of the underlying condition.
Table 29.1
Treatment options in lower gastrointestinal bleeding
Etiology | Treatment options |
|---|---|
Diverticular disease | 1.Resection ± anastomosis |
2.Angiography with embolization | |
Angiodysplasia | 1.Colonoscopy with hemostatic maneuvers |
2.Angiography with embolization | |
3.Resection ± anastomosis | |
Ischemic colitis | 1.Resuscitation and antibiotics |
2.Resection with diversion | |
Infectious colitis | 1.Resuscitation with antibiotics |
2.Resection ± anastomosis | |
Hemorrhoids | 1.Anoscopy with resection |
Neoplasm | 1.Resection ± anastomosis |
Radiation proctitis | 1.Intraluminal steroids |
2.Colonoscopy with hemostatic maneuvers |
Severe, persistent hemorrhage is the clinical presentation of lower gastrointestinal bleeding which most frequently requires surgical management. General indications for surgery include continued hemodynamic instability despite adequate resuscitation, requirement for transfusion of four or more units of packed red blood cells over 24 h, or severe recurrent bleeding [10]. Among patients who require a blood transfusion for the management of lower gastrointestinal bleeding, approximately one in four will ultimately require surgery [39]. The operative procedure of choice is a segmental resection for those patients in whom a hemorrhage source can be localized [40]. This approach is associated with greater control of bleeding and lower morbidity in comparison with the primary surgical alternative, a subtotal colectomy [7].
If efforts of localization are unsuccessful, as is the case in 8–12% of cases of acute lower gastrointestinal hemorrhage [25, 41], a subtotal colectomy is required to establish definitive control of bleeding [10]. Patients who undergo a total colectomy for control of lower gastrointestinal hemorrhage are at risk for considerable morbidity and mortality; overall mortality in this circumstance is between 10 and 20%, and those individuals with a transfusion requirement of ten or more units are subject to a mortality rate approaching 50%, likely mirroring the severity of underlying illness [42].
Given that a lower gastrointestinal bleed may result from a broad range of clinical conditions, the management of this patient population is dependent on the underlying diagnosis; however, there are general principles applicable to the management of all patients presenting with this clinical complaint.
Diverticular disease (Fig. 29.2) is the most frequently cited etiology for lower gastrointestinal bleeding in which a definitive source is identified, accounting for approximately 40–55% of all cases of acute lower gastrointestinal bleeding [2, 20]. The pathophysiology of bleeding due to diverticular disease is related to stretching and weakening of the vasa recta at the site of a colonic diverticulum. Diverticula are typically multiple. Diverticulosis is more commonly found in the left colon, in particular the sigmoid colon [10], but, curiously, diverticular bleeds are more commonly localized to the ascending colon [9]. Approximately one in six patients with diverticular disease will experience some degree of bleeding [10].
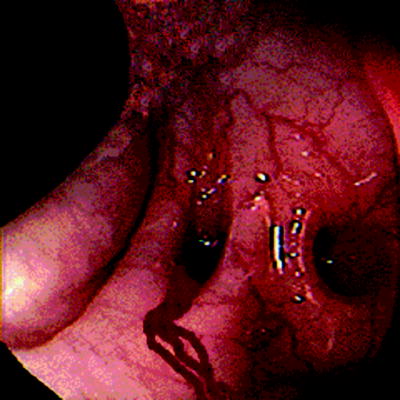

Fig. 29.2
Bleeding diverticulum
It is worth noting that lower gastrointestinal bleeding related to diverticular disease can occur within the setting of acute diverticulitis, but an acute episode of diverticulitis is by no means a prerequisite to bleeding. Although it might seem intuitive that the inflammatory changes associated with an episode of acute diverticulitis would increase the risk of acute hemorrhage, most diverticular bleeding occurs outside of acute diverticulitis. For unclear reasons, the hemorrhage is almost exclusively into the bowel lumen rather than into the extraluminal tissues [43].
Patients with acute diverticular hemorrhage present with painless, often brisk hematochezia, and in many cases, physiologic evidence of significant blood loss. Diverticular bleeding is highly unusual in patients under the age of 40, but the incidence rises with advancing age. The regular use of nonsteroidal anti-inflammatory drugs (NSAIDs) is also correlated with an increased likelihood of diverticular bleeding [44]. Ultimately, only a minority of patients with diverticular disease will experience bleeding, and of those patients who do, spontaneously resolution of bleeding occurs in approximately 75–80% [11, 45]. Re-bleeding is common, the rate of first re-bleed is estimated at 25–30%, and once this has occurred, the risk of subsequent re-bleeding is upwards to 50% [9].
The management of diverticular bleeding is dependent on several factors, including the severity of bleeding, whether or not the patient is experiencing a concurrent episode of acute diverticulitis, and the patient’s history of previous episodes of diverticular bleeding and/or diverticulitis [46]. A diverticular bleed in the absence of acute diverticulitis is generally well-suited to an initial attempt at evaluation and treatment via colonoscopy. If bleeding is ongoing and of sufficient rate, colonoscopy can localize the bleeding site and endoscopic treatments can be undertaken with a goal of achieving hemostasis. In patients with a history of recent diverticular bleeding who do not appear to be actively bleeding at the time of examination, colonoscopic evaluation is nonetheless worthwhile, because in many instances, the stigmata of recent bleeding, including adherent clots and visible vessels [10], are readily identified.
In the setting of acute diverticulitis, colonoscopy is generally contraindicated due to the acute inflammation and perforation associated with this diagnosis. For hemodynamically stable patients experiencing lower gastrointestinal bleeding concomitant with acute diverticulitis, the diverticulitis is the clinical priority in accordance with evidence-based standards of care. Milder cases are generally managed with a regimen of bowel rest, appropriate antibiotics, and serial abdominal examinations. More severe cases, especially those characterized by evidence of purulent or feculent peritonitis (i.e., Hinchey grade III or IV disease), are managed operatively. Surgical resection is also indicated for patients experiencing recurrent lower gastrointestinal hemorrhage due to diverticular disease. This represents a significant portion of patients with diverticulosis, with the incidence ranging from 10% at 2 years to 25% at 4 years [2].
If bleeding is severe in a patient with acute diverticulitis, angiography is a reasonable option for localization of the hemorrhagic site and establishment of hemostasis. In the event angiography is unsuccessful, surgical exploration is often required. Approximately 5% of patients admitted for diverticular bleeding ultimately require surgical intervention [47]. Such exploration may be performed via either laparoscopic or open approach based on surgeon preference and experience. Surgical resection is also the standard of care following a second significant diverticular bleed given the high (approximately 50%) risk of subsequent re-bleeding [48].
The question of primary anastomosis at the time of initial bowel resection depends in part on whether or not the patient is experiencing active and extensive diverticulitis-mediated inflammation; if such is not present, as is true in the majority of cases, primary anastomosis of the remaining viable bowel is generally deemed safe and appropriate. If active inflammation is present to a considerable extent, the surgeon may reasonably elect to perform a diverting ostomy with a plan for delayed anastomosis to take place once the acute inflammatory changes have resolved.
Angiodysplasia encompasses a broad range of lesions including arteriovenous malformations, vascular ectasias, and angiomas [9]. It is commonly considered in cases of lower gastrointestinal bleeding; however, its incidence is only 2.7% (hospital admissions for acute lower gastrointestinal bleeding, with age-specific bleeding rates showing a strong, positive correlation) [2, 49]. The pathophysiology is thought to relate to normal age-related degeneration of smaller venous structures located within the gastrointestinal submucosa. It is therefore seen predominantly in older patient populations. Boley and colleagues hypothesized that the lesions arise largely due to chronic, low-grade obstruction of the submucosal venous system [50]. The cecum is the most common site of angiodysplastic lesions [10]. There appears to be a possible correlation between angiodysplastic lesions and aortic stenosis and/or renal failure; however, there is no strong evidence to suggest a causative relationship [49, 51].
The bleeding associated with angiodysplastic lesions often presents as a history of intermittent, painless, bright red blood per rectum. In most circumstances, angiodysplasia-associated bleeding is subtle and may not be noted overtly by the patient. In these cases, the signs and symptoms of anemia may be the only evidence pointing to a gastrointestinal bleed, and angiodysplasia may be discovered as part of a broader workup. In approximately 15% of cases, however, angiodysplasia presents with significant hemorrhage [9]. Abdominal pain is infrequently associated with bleeding due to angiodysplasia, and a complaint of significant abdominal pain in a patient with known angiodysplasia should prompt a thorough workup for other diagnoses.
While angiodysplastic bleeding ceases spontaneously in roughly 90% of cases [41, 52], the majority of patients who present with one angiodysplastic bleed will bleed again, ultimately requiring a comprehensive evaluation [9]. Colonoscopy is the diagnostic and therapeutic modality of choice in the treatment of acute lower gastrointestinal bleeding due to angiodysplasia. The lesions have a characteristic stellate, bright red appearance on colonoscopic examination which facilitates identification. The right colon, in particular the cecum, is the most frequent site of bleeding angiodysplastic lesions [9, 50].
Angiography is sometimes used in the identification and treatment of bleeding angiodysplastic lesions. While angiography enjoys an overall greater diagnostic sensitivity in comparison with colonoscopy, it is thought by some authors to be less sensitive in identifying and treating the small venous lesions which are characteristic of angiodysplasia, while others cite increased sensitivity for angiography versus colonoscopy in this setting [53]. Overall, most patients with angiodysplastic bleeding are diagnosed and treated via colonoscopy. Endoscopic treatments include electrocautery, laser, and heater probe as well as the increasingly well-studied argon plasma coagulation (APC) technique. The APC technique appears to be well tolerated and is associated with fewer complications and lower risk of re-bleeding [8, 54]. Because of the documented explosive risk associated with APC in this setting, a complete bowel preparation is strongly recommended prior to utilization [55, 56].
In some instances, a patient with a history compatible with angiodysplasia-mediated lower gastrointestinal bleeding may present for evaluation between active bleeds, this may prove to be quite difficult or impossible. Patients should be warned that angiodysplastic lesions are likely to re-bleed in the majority of cases (up to 80% in some series) [52] and that timely evaluation in the event of a re-bleed may greatly increase the likelihood of successful identification and treatment of the lesion in question. Colon resection is generally employed as a last resort when recurrent angiodysplastic bleeding is unable to be controlled through colonoscopic treatment or angiography [7].
Bleeding secondary to colonic ischemia or hypoperfusion, termed ischemic colitis, is not infrequently encountered and should be entertained in the differential diagnosis for any patient presenting with acute lower gastrointestinal bleeding, particularly in those with abdominal pain and bloody diarrhea. “Pain out of proportion to the physical examination” is commonly associated with intestinal ischemia. In a large series of patients admitted for acute lower gastrointestinal bleeding, 8.7–11.8% of cases were ultimately attributed to ischemic colitis [2, 57]. Typically hemorrhage is a relatively minor component of the clinical presentation and blood loss is not of sufficient magnitude to independently affect hemodynamic stability [7]. Although acute mesenteric ischemia may present with a similar clinical picture, colonic ischemia is in fact considerably more common secondary to the relatively poorly collateralized vascular supply to the colon in comparison to the small intestine. Those areas with poorly collateralized vascular supply are at highest risk for colonic ischemia, namely, the ascending colon, splenic flexure, and rectosigmoid junction. Conventional wisdom has held that Griffith’s point is the single most common site of ischemic colitis, but rigorous investigation has failed to support this contention [58]. The diagnosis of ischemic colitis can be confirmed via colonoscopy with the characteristic findings including mucosal edema, erythema, mucosal necrosis, and hemorrhage with a clearly demarcated boundary between involved and uninvolved regions of bowel, reflective of the underlying vascular distribution [20, 59].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







