Long-Term Outcomes after Mechanical Ventilation: Introduction
When the first edition of this book was published in 1994, the standard metric of outcome after critical illness was 28-day all-cause mortality. Since that time, there has been a revolution in the way we view critical illness, its treatment, and its lasting effects both for the patient and the family caregiver. The dramatic rise in the number of longer-term outcomes reports published over the past 5 to 10 years clearly reflects this point. It is now apparent that most patients who have survived an episode of severe critical illness requiring mechanical ventilation will sustain some compromise in physical function related to intensive care unit (ICU) acquired weakness and a myriad of other physical disabilities.1–5 This acquired disability may be permanent.5 Patients may also sustain an important new or incremental decline in neuropsychological function, including neurocognitive impairments, and neuropsychiatric or mood disorders.6–8 The constellation of muscle, nerve, and brain dysfunction3,9–13 may permanently alter disposition for those who were previously independent and who, post-ICU, now require assisted living or comprehensive care.1 ICU-acquired morbidity may result in an additional cost burden in health care utilization that is similar to patients with chronic disease,1,5,13–14 and this traumatic life event may completely erode the reserve of family members and they may also acquire new mood disorders.15–18
This chapter reviews the literature on physical and neuropsychological outcomes after critical illness and highlights important emerging data that link patient characteristics, burden of comorbid disease, and ICU practice patterns to acquisition of new morbidity. In addition, newer data on psychological disability in family caregivers and models of rehabilitation and intervention after critical illness are discussed. To date, the outcomes literature has been dominated by reports on survivors of acute respiratory distress syndrome (ARDS), but there are emerging data on other vulnerable patient subgroups, such as the frail elderly and the chronically critically ill. Outcomes in these populations add insight to the current understanding of the diversity in post-ICU disability and also are discussed. The chapter concludes with a commentary on a proposed framework for a continuum of rehabilitation for patients and families after critical illness.
Physical Morbidity after Critical Illness
The current state of the outcomes literature suggests that the greatest short-term morbidity after critical illness is diminished physical health-related quality of life as a consequence of ICU-acquired weakness.3,5,9,10,13,19–22 Reports from long-term follow-up studies suggest that by 5 years, patients complain less of physical disability and more of a decline in general health, diminished vitality, and mood disorders.5 The finding of compromised physical quality of life has been robust across many studies since the early 1990s, as discussed in detail below. An understanding, however, of the specific determinants of this reported decrease in physical function was not well known until more than a decade later.
Patients with acute lung injury and ARDS represent some of the most complex critically ill and long-stay ICU patients and have been the most rigorously studied group of ICU survivors to date. More than 100,000 patients survive acute lung injury and ARDS every year24 and their outcomes dominate the review that follows. The emerging outcomes literature on the chronically critically ill, elderly, and sepsis patient populations adds nuance and depth to our understanding of the spectrum of disability and is also included.
Health-related quality of life is a set of causally linked dimensions of health, including biologic/physiologic, mental, physical, social function, neurocognitive, and health perception.21 Measures of health-related quality of life have emerged as important patient-centered metrics of recovery from critical illness. These questionnaires provide ease of administration and ready interpretability with published normative data for comparison, although fail to yield crucial details about the exact nature of any reported disability. Without this insight or more detailed understanding, the investigator is left to blindly hypothesize about the etiology of the reported disability and limits her or his ability to construct appropriate interventional or rehabilitation programs.
There is emerging evidence that the degree of disability acquired after critical illness and resultant health-related quality of life is variable and relates to differences in premorbid functional status, burden of comorbid illness, and nature and duration of critical illness.1,3,4 Although there is some heterogeneity across different study samples of patients with ARDS, there is less variability in reported health-related quality of life among this group than in general populations of critically ill patients.24 The following is an overview of the literature on ARDS health-related quality of life and these data highlight the consistent signal of diminished physical function reported up to 1 year after severe lung injury, and also the controversy that still continues about the relative contribution of pulmonary disability to functional decline after ARDS and severe critical illness. In 1994, McHugh et al were the first group to prospectively evaluate the relationship between pulmonary dysfunction and functional disability in patients with ARDS.19 They noted that scores for the Sickness Impact Profile (a generic quality-of-life measure of the subject’s self-perceived physical and psychological condition) rose substantially to 3 months postextubation with only minimal further improvement to 1 year. Using a lung-related Sickness Impact Profile score, only a modest proportion of the patients’ overall physical dysfunction was attributed to pulmonary difficulties. Weinert et al20 documented functional impairment in a cohort of lung injury survivors using the Medical Outcomes Study 36-item short-form health survey (SF-36) and reported the largest decrements in role-physical and physical-functioning domains, which patients attributed to global disability.25 Similar observations were made by other groups who inferred that disability in their patients with ARDS was secondary to pulmonary dysfunction in the absence of any direct pulmonary measurement26 or through administration of pulmonary disease-specific measures and this failed to differentiate between pulmonary and extrapulmonary disability.22 Others have made comparisons to control populations of patients with known pulmonary disease (e.g., cystic fibrosis), but after health-related quality-of-life measures had been disaggregated, symptom burden was seen to be related to musculoskeletal or constitutional issues.10 In the context of outcome evaluation after a trial comparing higher versus lower tidal volumes during mechanical ventilation, some have demonstrated very modest abnormalities of pulmonary function, which correlate with reduced function on health-related quality-of-life metrics, and maintain that there is an important relationship between pulmonary and functional outcomes in ARDS survivors.28
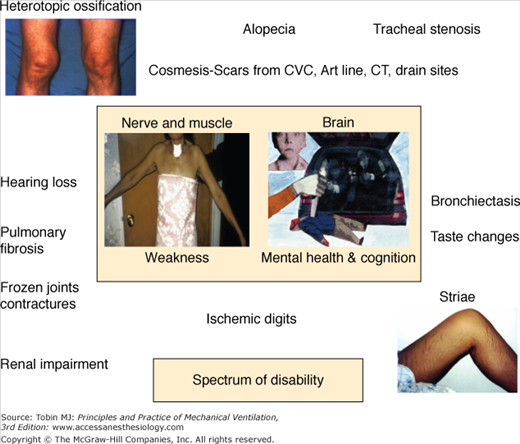
In 2003, Herridge et al reported on 109 ARDS survivors at 3, 6, and 12 months after ICU discharge and captured function as distance walked in 6 minutes, pulmonary function, and health-related quality of life using the SF-36. The investigators also did a detailed in-person interview and physical assessment as part of each follow-up visit. In their relatively young patient sample (median age: 45 years), with few comorbidities and who had been working full time before their critical illness, 6-minute walking distance was 66% at 1 year and pulmonary function was normal or near normal. They noted that the major morbidity after ARDS and sepsis was muscle wasting and weakness.3 In-person evaluation of patients and the collection of multiple outcome measures led the investigators to different conclusions about the major contributors to morbidity after severe lung injury and also facilitated the generation of a catalogue of physical disabilities that contribute to poor health-related quality of life (Fig. 68-1).
Impaired physical function is a robust observation across different studies, countries, and investigators, and may persist for years after ICU or hospital discharge and be irreversible (Fig. 68-2).4,5 Health care costs generated by relatively young, previously working patients in an ARDS cohort followed to 5 years were higher than predicted for this age group and comparable to patients with chronic disease (Fig. 68-3). It was not expected that this patient group would not fully recover from their critical illness in terms of physical functioning, emotional outcomes, or quality of life, and this finding has significant public health ramifications.
Figure 68-2
Top Panel: Survival—Kaplan-Meier curve to 5 years. Dashed lines represent the 95% confidence interval. Exact survival times were used for these analyses. Middle Panel: Six-minute walk distance—Distance walked in 6 minutes (meters and % predicted); distance in meters is a solid line and % predicted is a dashed line.Bottom Panel: Quality of life to 5 years after ICU discharge—SF-36 Subscale scores for Physical Component Score (PCS) and Mental Component Score (MCS). (Used, with permission, from Herridge et al.5)
Figure 68-3
Cumulative costs to 5 years after ICU discharge stratified by number of comorbid conditions at ICU admission. Lower line is less than or equal to one comorbid condition and upper line is greater than or equal to two comorbid conditions. (Used, with permission, from Herridge et al. N Engl J Med. 2011.5)
The ARDS outcomes data are instructive because most of these study samples involved younger patient groups with few or no premorbid conditions. It was a surprising and striking observation that these patients did not fully recover and had important and persistent morbidity for years after their critical illness. These data establish that there is a physical and neuropsychological “cost” to a severe episode of critical illness, and that most patients, particularly those in more vulnerable groups, such as patients requiring prolonged mechanical ventilation, patients with a significant burden of comorbid illness, and the elderly, may suffer even greater functional decline and require dependent care as a result of their critical illness.
Studies on survivors of prolonged mechanical ventilation have helped in elucidating important risk factors for outcome and change in disposition. Chelluri et al evaluated factors associated with mortality and quality of life in 817 patients 1 year after prolonged mechanical ventilation.28 The median age of their patients was 65 and 1-year survival was 44%. Those patients surviving their ICU stay had fewer comorbidities, lower severity of illness scores, and less premorbid dependence in activities of daily living. Fifty-seven percent of surviving patients needed caregiver assistance at 1 year of follow-up. A French study by Combes et al evaluated 347 patients receiving mechanical ventilation for equal to or greater than 14 days.29 Factors associated with death in the ICU included age greater than 65 years, preadmission New York Heart Association functional class of equal to or greater than 3, preadmission immunocompromised status, septic shock at ICU admission, need for renal replacement therapy in the ICU, and nosocomial septicemia.
Unroe et al evaluated the trajectories of care and resource utilization for 126 patients with a median age of 55 years who also required prolonged mechanical ventilation. These patients had, on average, two comorbid conditions, and most were not working, retired, or disabled at the time of ICU admission. At 1 year, only eleven patients (9% of the cohort) were alive and without functional dependency. Patients with poor outcomes were older, had more comorbid conditions, and were more frequently discharged to a post–acute care facility. The mean cost per patient was $306,135 (standard deviation [SD]: $285,467) and total cohort cost was $38.1 million, for an estimated $3.5 million per independently functioning survivor at 1 year.1
Iwashyna et al noted persistent reduction in functional status after sepsis and critical illness, again echoing the theme of acquired disability after critical illness. Among older patients (median age: 77 years), they observed a high rate of new functional limitations in those who had no limits before their episode of sepsis (mean: 1.57; 95% confidence interval [CI]: 0.99 to 2.15). In patients with reductions in activities of daily living before sepsis, they noted an important further decrement in function. Neurocognitive and physical decline persisted for at least 8 years after the episode of sepsis and altered patients’ ability to live independently.4
The impact of increased age, however, on outcomes after critical illness remains somewhat controversial. Some studies report higher mortality with advanced age30 and others do not.31,32 Khouli et al33 recently evaluated 484 patients, who were age 65 years and older, and administered an health-related quality-of-life instrument to the patient or proxy at ICU admission and 6 months after hospital discharge. One-third of these patients died within 6 months of hospital discharge. Independent predictors of death at 6 months were: number of days during the 30 days before hospitalization that the patient felt their “physical health was not good,” a higher Acute Physiology and Chronic Health Evaluation (APACHE) II score, and chronic pulmonary disease. This same group also found that the oldest survivors (age 86 years) had worse health-related quality of life over time, including more days spent with poor physical and mental health, compared to baseline. There appears to be a clear prognostic signal in the elderly in terms of physiologic reserve, burden of chronic organ dysfunction, and nature of the health trajectory before the critical illness with regards to prognostication for survival, function, and health-related quality of life; emphasis on physiologic rather than chronologic age may provide valuable insight into projected outcome.
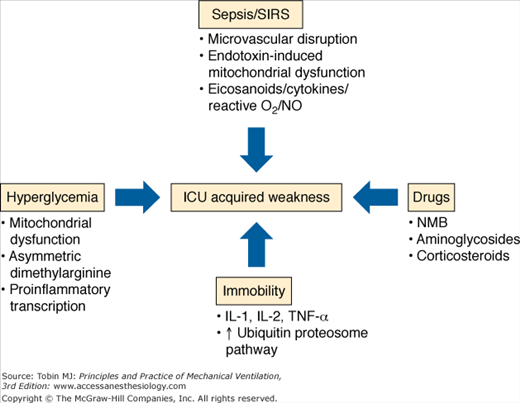
Muscle weakness and impaired function constitute an important morbidity of severe critical illness. A continuum of weakness begins within hours of mechanical ventilation,34 is demonstrable by bedside evaluation within 1 week of ICU admission using the Medical Research Council scoring system,35 and may persist with incomplete recovery for years after ICU discharge5,36–41 (see Fig. 68-2). In the ICU context, muscle weakness has been linked to prolonged mechanical ventilation,42,43 delay in ICU and hospital discharge, increase in associated costs,44,45 and increased likelihood of death.46
Many challenges confound the ability to characterize and intervene in neuromuscular disability after critical illness. Important heterogeneity exists in susceptibility and risk factors across different critically ill patient populations. There are no validated measures to reliably risk stratify patients according to physical disability and no consistent nomenclature for the spectrum of lesions that constitute muscle weakness and wasting. Currently, different terminologies coexist in the literature and include critical illness polyneuropathy, critical illness polyneuropathy and myopathy, critical illness neuromyopathy, ICU-acquired paresis, critical illness myopathy and/or neuropathy. This complexity is compounded by the observation that nerve and muscle injury may coexist, and it is difficult to delineate discrete risk factors and natural history. There are obstacles in terms of the variety and sensitivity of testing methods, criteria for diagnosis, surveillance and selection bias in evaluation and reporting. Sensory and motor evaluations are limited in heavily sedated patients, and, therefore, clinical bedside testing and determination of the prevalence of these lesions may be unreliable.
Despite these limitations, a recent classification system was proposed by Stevens et al (Table 68-1).47 This system is simple, accessible, and clinically relevant, and emphasizes clinical assessment and bedside testing. It proposes a broad classification for ICU-acquired weakness and highlights it as a diagnosis of exclusion, onset during the critical illness, ventilator dependence, and weakness demonstrable by use of bedside hands-on muscle testing (Medical Research Council score). A diagnosis of critical illness polyneuropathy is made in those who meet criteria for ICU-acquired weakness and have electrophysiologic evidence of a sensorimotor axonal polyneuropathy. A diagnosis of pure critical illness myopathy is made in patients who meet criteria for ICU-acquired weakness and who have myopathic features on electromyelography during muscle contraction and/or myopathic biopsy.
Diagnostic criteria for ICU-acquired weakness (ICUAW) 1, 2, 3 or 4, 5 below
|
Probable CIM: criteria 1, 2, 3, or 4; or 1 and 5 (listed above) Definite CIM: criteria 1, 2, 3 or 4, 5 (listed above) |
This section discusses the pathophysiologic mechanisms for muscle and nerve injuries (Fig. 68-4). Given the limitations in this distinction, as outlined above, the mechanisms are broadly grouped as critical illness polyneuropathy and critical illness myopathy.
Bolton et al published a landmark study on critical illness polyneuropathy in 1984.48 They described a primary axonopathy that presented as a mixed sensorimotor neuropathy in five critically ill patients who were ventilator dependent. Detection of the true incidence of critical illness myopathy is complicated by poor consensus on surveillance, timing, and nature of testing, and formal definition and diagnostic criteria (as outlined above). When patients are evaluated solely on the presence of clinical weakness, studies demonstrate an incidence of 25% to 36%.35,49 A review of 1421 critically ill patients, evaluated using diagnostic tests (nerve conduction velocities, needle electromyography, direct muscle stimulation, histopathology of muscle or nerve tissue) or a combination of test findings and clinical findings, reported an incidence of critical illness neuromyopathy of 46% (95% CI: 43% to 49%).50
Critical illness polyneuropathy has been associated with sepsis and systemic inflammatory response syndrome in multiple cohort studies.50 In sepsis, critical illness polyneuropathy is linked to microcirculatory perturbations with resultant axonal injury and degeneration. A recent report describes proinflammatory cytokine (tumor necrosis factor-(and interleukin-1)-mediated increased expression of E-selectin on the endoneurial and epineurial vessels of peripheral nerves.51 There may be functional disruption of nerve action potential early in the course of disease, which may be a harbinger of later structural derangement of the nerve.52
Hyperglycemia in critically ill surgical and medical populations is consistently associated with ICU-acquired weakness.53,54 Van den Berghe et al demonstrated that tight glycemic control reduced critical illness polyneuropathy in defined by neurophysiologic testing, from 51.9% in control subjects to 28.7% among insulin-treated patients.53,54 Hyperglycemia may be harmful based on mitochondrial dysfunction and the deleterious effects of oxidant injury and apoptosis.55,56 Derangement of nitric oxide production is also a postulated mechanism,57 as is the role of insulin in inhibiting proinflammatory transcription factors and promotion of neuroregeneration during critical illness.58,59
Early reports suggested an association between neuromuscular dysfunction and exposure to neuromuscular blockers and systemic corticosteroids.60–62 This risk was profiled in patients with status asthmaticus who developed ICU-acquired weakness and received treatment with both agents.63 These risks have not been borne out in systematic review because of confounding by exposure to aminoglycosides, vasopressors, and renal replacement therapy in sepsis and systemic inflammatory response syndrome patients.50,64–65 In a 1-year outcomes study in patients with ARDS, exposure to any systemic corticosteroid was significantly associated with a decrease in 6-minute walking distance at 3 months in a multivariable model, and patients demonstrated important proximal weakness in the shoulder and hip girdle at that time point.3 A recent trial using 48 hours of continuous paralytic in patients with severe lung injury saw significant organ function improvement, and the continuous paralytic was not associated with an increased risk of ICU-acquired weakness assessed by manual muscle testing at ICU discharge.66 No long-term follow-up, however, was done, and there is concern that manual muscle testing may not have adequate sensitivity to detect ICU-acquired weakness.66 Early corticosteroid use appears to be strongly associated with ICU-acquired weakness, although the risk associated with paralytic use remains somewhat uncertain.
Critical illness myopathy currently encompasses critical illness myopathy, acute quadriplegic myopathy, thick filament myopathy, and necrotizing myopathy, and the incidence varies between 48% and 96% in studies that included muscle biopsy.65 Critical illness myopathy is a nonnecrotizing diffuse myopathy associated with fatty degeneration of muscle fibers, fiber atrophy, and fibrosis.67 This lesion has also been linked to corticosteroid and paralytic exposure and may be clinically indistinguishable from critical illness polyneuropathy because patients are also weak, paretic, and difficult to wean.
Thick-filament myopathy shows a selective loss of myosin filaments in the context of significant corticosteroid or neuromuscular blocker exposure and immobility.68 Acute necrotizing myopathy is distinguished by extensive myonecrosis with vacuolization and phagocytosis of muscle fibers, and is linked to corticosteroid and neuromuscular blocker exposure and multisystem organ dysfunction.69
The pathophysiology of critical illness myopathy involves catabolism, inflammation, and derangement of membrane excitability. An increase in urinary nitrogen loss, low glutamine, protein, and DNA levels in muscle biopsies, and upregulation of the calpain and ubiquitin proteolytic pathways and apoptosis have all been documented.70
Inactivity in critically ill patients propagates inflammatory mediators, which stimulate protein loss in differentiated muscle cells, activate signaling events that promote oxidative injury and disruption of insulin receptor signaling with resultant substrate reduction and impairment of myofibril growth and repair.71 Interleukin-1, interleukin-6, and tumor necrosis factor-α have proinflammatory properties and have all been implicated in muscle degradation in critical illness and augmentation of proteolysis and muscle loss. Muscle membrane inexcitability may also contribute to weakness and is related to inactivation of sodium channels. Allen et al recently reported altered muscle-fiber excitability and evidence for muscle membrane dysfunction as the principal underlying abnormality in critical illness myopathy.72
Pulmonary function outcomes may be heterogeneous after an episode of ARDS. Many ARDS survivors have persistent pulmonary function impairments: typically, mild restrictive changes with an associated reduction in diffusion capacity.3,19 Others have documented more diversity in outcome, with significant variability in the proportion of patients with obstructive (0% to 33%) and restrictive (0% to 50%) defects, as well as compromised diffusion capacity (33% to 82%).73 There are reports of mild pulmonary function abnormalities associated with decreased health-related quality of life 1 year following hospital discharge27 and no improvement in pulmonary function after the first year.74 Most recent data from a 5-year outcomes cohort study in patients with severe ARDS shows normal to near normal pulmonary function achieved by 6 months to 1 year after ICU discharge and continued stability over the 5-year study period.5
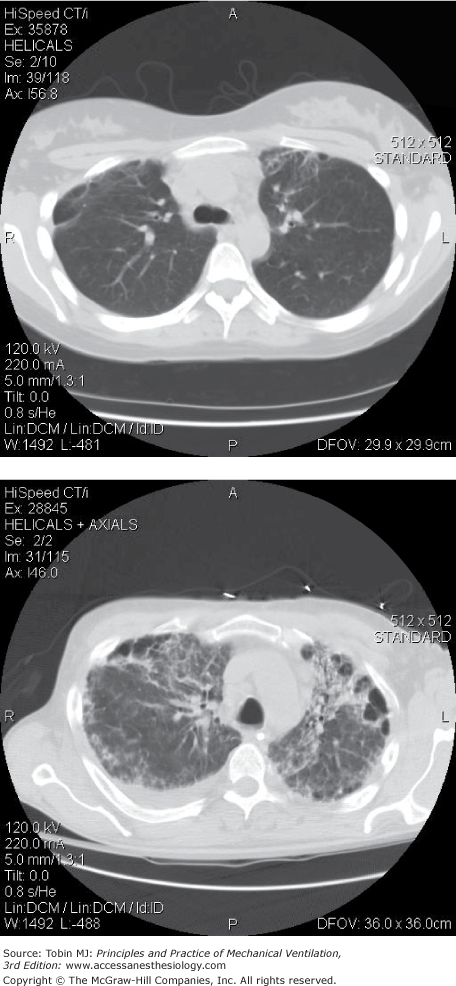
There are imaging studies of ARDS survivors at 6 to 10 months,75 3 years,76 and 5 years.5,77 Investigators have noted that most patients with ARDS had minor localized changes in the nondependent lung zones (Fig. 68-5), and were able to document some association with severity of lung injury and duration of mechanical ventilation.75,76 There were also findings of bronchiectasis or new pulmonary fibrosis in one-third of patients in one study5 (Fig. 68-5) and loss of ventral distribution in reticular pattern in patients treated with extracorporeal membrane oxygenation.77
Figure 68-5
Top Panel: Computed tomography (CT) findings at 1 year after acute respiratory distress syndrome (ARDS) in a young, previously healthy person without preexisting lung disease. Finding of minor nondependent fibrosis and minor cystic changes were most common in this group. Bottom panel: CT findings at 1 year after ARDS in same group of ARDS survivors with an example of a rare finding of more marked nondependent fibrosis, bronchiectasis, and cystic changes.
The variability in reported pulmonary outcomes to date suggests possible confounding by smoking history, preexisting obstructive or restrictive pulmonary disease, physiologic restriction related to ICU-acquired weakness affecting respiratory muscles, presence of other pulmonary processes that fulfill the ARDS definition but that have a very different natural history (e.g., cryptogenic organizing pneumonia), and loss to follow-up that continues to challenge validity and interpretation of follow-up data. Most outcome studies found ARDS survivors are often unable to resume their prior physical function, and the degree of pulmonary dysfunction documented across studies does not solely explain their degree of functional limitation.
The spectrum of physical morbidities after critical illness is shown in Figure 68-1.
The Toronto ARDS Outcomes study observed a 6% prevalence of peroneal and ulnar nerve palsies.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree