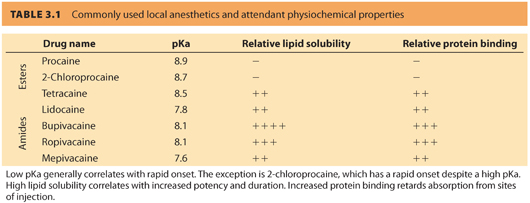
B. Local anesthetics with an ester bond linking the aromatic ring to the hydrocarbon chain are classified as amino-ester local anesthetics. These include procaine, 2-chloroprocaine, tetracaine, and cocaine (see Table 3.1). The amino-amide local anesthetics contain an amide bond between the aromatic ring and the hydrocarbon chain. Common amino-amides include lidocaine, bupivacaine, ropivacaine, and mepivacaine.
C. Amides, except lidocaine, have chiral centers with the levorotary isomer of bupivacaine (levobupivacaine, currently not marketed in the United States) conveying greater vasoconstrictor properties and less toxicity than either its racemic mixture (used commonly in clinical practice) or its dextrorotatory form.2 Ropivacaine is available in its levorotary form only.
II.Mechanism of action. Lipid solubility, pKa, and protein binding all determine the clinical profile of local anesthetics. Local anesthetics act on the sodium channel by reversibly binding to the intracellular portion of voltage-gated sodium channel that, in turn, disrupts action potentials along the nerve fibers.
A. Local anesthetic entry into the cell. Only the uncharged form of the local anesthetic molecule readily enters and/or crosses the cell membrane.3
1. Commonly used local anesthetics are weak bases that have a pKa greater than physiologic pH. (A base is defined as a molecule capable of accepting a proton, and pKa is defined as the pH at which 50% of the molecules will be in the protonated form.)
2. When local anesthetics are administered at physiologic pH, more than 50% of the molecules become protonated at the terminal amine. These molecules exist in the ionized form and are unable to enter or cross the cell membrane due to their charge.
3. Local anesthetics are marketed as water-soluble salts, usually hydrochlorides, in order to increase their solubility; therefore, most preparations are acidic, which further increases the protonated ionic form of the molecule.
4. The pKa of any specific local anesthetic partially determines its speed of onset, in that a molecule with a pKa closer to physiologic pH will be less ionized and thus have a faster speed of onset than a similar molecule with a higher pKa.
5. An increased ratio of lipid soluble form to the protonated form of the local anesthetic facilitates drug entry into the cell membrane. Thus, increasing lipophilicity is associated with increasing local anesthetic potency.3
6. Increased protein binding retards absorption from sites of injection, thus lowering blood levels of those local anesthetics.4
CLINICAL PEARLThe clinical profile of a local anesthetic is determined by its lipid solubility, pKa, and protein binding characteristics.
B. Local anesthetic binding to the sodium channel
1. Once the molecule has entered the cell membrane, the protonated form enters the voltage-gated sodium channel and reversibly binds at a specific binding site on its inner pore.5
2. Binding occurs more readily when the sodium channel is in the activated or the deactivated state (i.e., the states associated with depolarization) rather than the resting state. This results in a phasic block (sometimes called use-dependent or frequency-dependent block): a block that increases with repetitive depolarizations and represents increased local anesthetic binding with repetitive depolarizations.
3. Binding interferes with the conformational changes of the sodium channel that are necessary for activation and thus prevents the passage of sodium ions that is necessary for generating an action potential.3
C. Local anesthetic dissociation from binding site
1. Dissociation from the binding site involves a complex interaction of molecular size, charge, and lipid solubility.
2. Smaller local anesthetics dissociate from the sodium channel binding site more rapidly than larger molecules. Moderate lipophilicity aids departure of local anesthetics from the binding site, but extreme lipophilicity (e.g., bupivacaine) favors continued binding and increases duration of action.6
D.Local anesthetic effects on other membrane-bound proteins. Local anesthetics probably affect many other membrane-bound proteins in addition to sodium channels, especially during epidural and spinal anesthesia, and this may contribute to their effects. These proteins include adenylate cyclase, guanylate cyclase, sodium/potassium ATPase, calcium/magnesium ATPase, and potassium channels.3
III.Differential blockade
A. Physiologic basis. Not all sensory and motor nerves are blocked equally by local anesthetics. Differential blockade refers to the observed tendency of different types of nerves to demonstrate different susceptibilities (i.e., successive disappearance of temperature sensation, proprioception, motor function, sharp pain, and light touch) to local anesthetic–mediated conduction blockade.
1. See Table 3.2 for a list of the different types of nerve fibers present in humans, along with data regarding axon diameter, presence or absence of myelination, and function of each type of nerve. Presence of a myelin sheath and larger nerve size result in faster conduction velocities. Large-diameter, myelinated fibers (e.g., A fibers) are mostly involved in sensory and motor functions. Speed of nerve transmission is critical for these functions. Unmyelinated small-diameter C fibers have slower conduction velocities and relay sensory functions (e.g., pain, temperature, and autonomic functions).
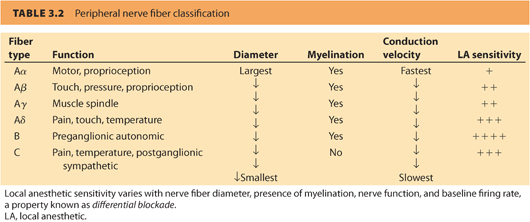
2. Presence or absence of a myelin sheath differentiates nerves in the central and peripheral nervous systems. Myelinated axons are more sensitive to local anesthetic blockade than unmyelinated axons because the myelin sheath has interruptions (i.e., nodes of Ranvier) that facilitate conduction so that an action potential is generated. Unmyelinated fibers require more local anesthetic exposure for similar degrees of nerve conduction blockade to result.6
3. In general, axons of smaller diameter are more sensitive to local anesthetic action than larger diameter axons. For example, local anesthetics produce an orderly progression of loss of temperature sensation, followed by proprioception, motor function, sensation, and light touch. This may not be a function of size per se but may reflect the fact that large-diameter nerves may have longer distances between nodes of Ranvier than small-diameter nerves. When there is a longer spacing between nodes, a longer critical length of nerve exposure to local anesthetic may be required before blockade occurs.6
4. Axonal size corresponds with different nerve cell function. Blockade that appears to vary according to diameter may actually reflect variation according to anatomic and physiologic differences among nerves with different functions, such as differences in density and gating of ion channels, differences in myelination, differences in the density of sodium/potassium ATPase or other ion pumps, and so on.7
5. Conduction blockade characteristics may also be a function of the location of a nerve fiber within a large nerve trunk.
6. Phasic block dictates that nerves with higher baseline firing rates will demonstrate greater blockade sensitivity than nerves with lower firing rates. Preganglionic sympathetic vasomotor nerves may be more susceptible to phasic block because they have a tonic vasoconstrictor function and a high basal rate of firing. Similarly, because sensory nerves tend to fire at increased rates compared to motor nerves, phasic block may be stronger in sensory than motor nerves.
CLINICAL PEARLDifferential blockade refers to the observed tendency of different types of nerves to demonstrate different susceptibilities (i.e., successive disappearance of temperature sensation, proprioception, motor function, sharp pain, and light touch) to local anesthetic–mediated conduction blockade.
B. Anesthetic implications
1. Sympathetic blockade exceeds sensory blockade by several dermatomes during spinal and epidural anesthesia, although sympathetic block is not always complete.8–10
2. Sensory blockade exceeds motor blockade during spinal and epidural anesthesia. Epidural labor analgesia with low concentrations of local anesthetics can be used to provide pain relief with minimal effects on maternal expulsive efforts.11
3. The Aδ fibers, associated with sharp or fast pain, demonstrate more susceptibility to local anesthetic effects than C fibers, associated with burning or slow pain.7
4. Cold temperature–sensing fibers demonstrate more conduction blockade than pain-sensing fibers.12,13 The degree of differential blockade is dependent on the concentration of local anesthetic and volume. The use of less concentrated solutions and greater volume often produces less motor block but a more uniform sensory block.
5. A sensory level to pain, as opposed to temperature sensation, should be used to gauge the adequacy of sensory blockade during regional analgesia/anesthesia.
IV.Additives. Additives are combined with local anesthetics to achieve various clinical effects.
A. Bicarbonate. As noted earlier, commonly used local anesthetics are ionized because they are weak bases with pKa higher than physiologic pH. Addition of bicarbonate to the local anesthetic solution (1 mEq/mL) (8.4%) adjusts local anesthetic pH closer to pKa and increases the ratio of the lipid-soluble form to the protonated form. This results in less ionization, facilitates drug entry, and hastens speed of onset.
B. Epinephrine. Epinephrine is an important additive to increase local anesthetic activity. Besides intensifying local anesthetic–induced anesthesia and analgesia, epinephrine prolongs the duration of the block and reduces the systemic absorption of the local anesthetic. Greater reliability and intensity of the block are observed when epinephrine is added to epidurally administered local anesthetics. Epinephrine has intrinsic analgesic effects that are similar to drugs (e.g., clonidine) which produce analgesia by stimulation of α2-adrenergic receptors in the spinal cord. Although epinephrine has a bupivacaine dose-sparing effect when used as an additive for epidural labor analgesia,14 it is associated with increased motor block. Epinephrine causes vasoconstriction and thus reduces clearance of drugs from the intrathecal and epidural spaces into the central circulation.15 Epinephrine reduces mean peak plasma concentrations of epidurally administered lidocaine and bupivacaine, which is significant when considering the potential for local anesthetic toxicity.16 Intrathecal epinephrine is administered in doses between 50 and 200 μg and epidurally in doses of 1 to 5 μg per mL of local anesthetic.
CLINICAL PEARLWhen epinephrine is added to lidocaine, it improves the density as well as prolongs the duration of the block and reduces the systemic absorption of the local anesthetic.
C. Phenylephrine. Phenylephrine as a vasoconstricting additive has fallen out of favor due to an increased incidence of transient neurologic symptoms (see following text) associated with its use.17
D. Opioids. Opioids are often added to local anesthetics administered in the spinal or epidural spaces and have synergistic effects without evidence of toxicity.
V. Effect of pregnancy on local anesthetic action. Pregnancy enhances local anesthetic effects. Pregnancy alters local anesthetic neural blockade, susceptibility to toxicity, and pharmacokinetics. Pregnant women typically require smaller doses of local anesthetic compared to nonpregnant women. Long-acting amide local anesthetics, such as bupivacaine, are beneficial for labor neuraxial analgesia because they produce a relative motor-sparing block as compared to other local anesthetics. The motor-sparing effect appears to be enhanced during pregnancy. For instance, the ED50 for motor block after intrathecally administered bupivacaine was lower in pregnant women as compared to nonpregnant ones, 3.96 and 4.14, respectively.18 These effects may be evident as early as the second trimester.19,20 Although the difference has been attributed to enhanced spread of local anesthetic due to epidural venous engorgement, mechanical effects alone do not account for the observation that the spread of spinal and epidural analgesia in early pregnancy is similar to that in pregnant women at term.
A. High spinal or epidural block accounted for 16% of anesthesia-related pregnancy deaths from 1992 to 2002.21
B. Increased cephalad spread during pregnancy is due to both mechanical and nonmechanical factors.
1. Mechanical compression of the vena cava by the gravid uterus leads to distension of the epidural veins, which in turn decreases intrathecal volume.20 Therefore, equal doses of local anesthetics may result in higher anesthetic levels in pregnant compared to nonpregnant patients.18
2. Nonmechanical factors: Pregnancy increases median nerve sensitivity to lidocaine block.22 In vitro preparations from pregnant versus nonpregnant animals demonstrate increased susceptibility to bupivacaine and lidocaine conduction blockade.23–25 Anatomic differences alone do not account for these observed peripheral and in vitro differences. These differences are due to progesterone and/or other hormonal mediators that affect membrane excitability, increase permeability of the neural sheath, and/or potentiate the analgesic effect of endogenous opioids.18,19,26
C. Cerebrospinal fluid has a greater pH and lower PCO2 during pregnancy. These physiologic changes can favor diffusion of the non-ionized form of the local anesthetic across the nerve cell membrane. However, it is not completely understood what effect this may have on cephalad spread of analgesia.
D. It is not known when local anesthetic requirements return to normal, but patients for postpartum tubal ligation require a higher dose of intrathecal bupivacaine per blocked segment 36 to 48 hours after delivery compared to patients undergoing cesarean delivery.27
CLINICAL PEARLEqual doses of local anesthetics result in higher neural blockade in pregnant compared to nonpregnant patients due to mechanical and hormonally mediated factors.
VI. Pharmacokinetics. An understanding of the pharmacokinetics of local anesthetics requires knowledge of the principles of absorption from injection site, distribution, and clearance.
A. Systemic absorption
1. Kinetic variables relevant to a discussion of absorption include the maximal blood concentration (Cmax) that occurs after perineural injection and the time at which Cmax occurs.
2. Systemic absorption from the site of injection is dependent on local blood flow and local tissue binding such that drugs are absorbed more rapidly from highly vascular sites such as the epidural space than from peripheral nerve or subcutaneous sites. However, the level at which the epidural space is entered does not affect absorption.4,28 Vasoconstrictors reduce Cmax and increase mean absorption time. These effects are greatest in highly vascular tissues such as the epidural space.16 Rate of absorption slows for drugs that are highly bound to local adipose tissues (which depends on drug lipid solubility) or local tissue proteins (which depends on drug protein binding strength).4,29
3. A biphasic pattern of absorption exists due to relatively rapid absorption from the aqueous phase but a delayed slower absorption from adipose tissue.4
B. Distribution. Volume of distribution depends on the degree of plasma and blood cell versus tissue binding of drug. The volume of distribution is smaller for highly protein-bound drugs (e.g., bupivacaine). Enantiomeric differences in volume of distribution exist due to differences in plasma protein binding; levobupivacaine is more highly protein bound and has a smaller volume of distribution than racemic bupivacaine.30
C. Clearance
1. Ester-type anesthetics are hydrolyzed by plasma esterases, including pseudocholinesterase, red cell, and liver esterases. Hydrolysis occurs within a few minutes in both the mother and the fetus.4,29 One exception is cocaine because it is slowly metabolized by the liver. It is generally not used in obstetrics due to its vasoconstrictive effects.
2. Clearance of amide-type local anesthetics occurs almost exclusively in the liver.
a. Lidocaine has a high hepatic extraction ratio (70% to 75% first pass), and thus, its clearance is largely dependent on hepatic blood flow, which can be reduced during epidural anesthesia and with alterations in protein binding. Other factors that limit hepatic blood flow (e.g., volatile anesthetics, congestive cardiac failure, intravascular volume depletion) reduce clearance.
b. Conversely, bupivacaine and ropivacaine have more intermediate hepatic extraction ratios (<50% first pass), so clearance is more dependent on free drug concentration and intrinsic enzymatic activity.4
c. Patients with renal dysfunction or severe cardiac disease also have reduced clearance of local anesthetics and require dose reduction of medication during repeated dosing or continuous infusion. Uremia causes a hyperdynamic circulation that rapidly increases the plasma concentration of bupivacaine and ropivacaine; however, a concomitant increase in α1-acid glycoprotein is protective, binding toxic levels of unbound local anesthetic.29,31
D. Elimination. Drug elimination is offset by continued systemic absorption, so measured serum half-lives are longer after epidural than intravenous administration.32 Because elimination occurs at a faster rate than absorption after epidural administration, mean residence time (the mean time drug molecules remain in the body) may be a more meaningful measure than elimination half-time.
E. Amide protein binding. The amides bupivacaine, ropivacaine, and levobupivacaine are highly protein bound to α1-acid glycoprotein.33
F. Local anesthetic continuous infusions. During continuous bupivacaine or ropivacaine infusion, a slow rise in serum concentration of drug occurs over time.34,35 This is offset by increases in α1-acid glycoprotein concentrations that occur in the postoperative population, leading to increased protein binding, such that concentrations of free drug remain unchanged over time.36,37
G. Effect of pregnancy on pharmacokinetics. During pregnancy, volumes of distribution of bupivacaine and ropivacaine are lower; however, clearance is also decreased so that serum half-life of elimination and mean residence time are unchanged.33,38 Therefore, following inadvertent intravascular injection in a pregnant patient, one might expect a higher peak serum concentration but normal elimination.
H. Chronobiology. Diurnal neuroendocrine or other external factors may affect pain sensitivity. For example, in one study, the duration of action of epidural ropivacaine was approximately 20–28% longer in the diurnal period (morning and afternoon) when compared with the nocturnal period (evening and night).39 In a more recent study, the peak effect of labor analgesia occurred between 2:00 AM and 5:59 AM. Some have suggested that these differences may be explained by the fact the chronobiology may also be affected by such influences as shift change for nurses and anesthesiologists.40
VII. Local anesthetics rapidly cross the placenta.38
A. Although the total drug concentration is lower in the fetal than in the maternal circulation, the fetus possesses lower concentrations of α1-acid glycoprotein, so that fetal and maternal free drug concentrations are approximately equivalent.4,41
B. When a fetus is acidotic, ion trapping may occur because local anesthetics may be relatively more ionized than maternal blood. This can lead to fetal drug accumulation.
VIII. Systemic toxicity from high circulating plasma levels of local anesthetics can occur after unintentional intravenous injection or from absorption of local anesthetic after neural blockade. In most cases, larger doses of local anesthetics are required to produce cardiovascular toxicity compared to doses producing central nervous system toxicity. Unintentional venous cannulation and injection are more likely to occur during epidural anesthesia in the pregnant patient, secondary to engorgement of epidural veins. Because blood levels rise rapidly, direct intravascular injection is associated with rapid onset of seizure activity. In contrast, toxicity that occurs due to absorption of local anesthetic resulting from epidural blockade is associated with a 20- to 30-minute delay in onset of symptoms. Blood concentrations of local anesthetics may remain elevated for longer periods of time than after unintentional intravenous injection.42 See Table 3.3 for symptoms of local anesthetic systemic toxicity (LAST).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







