Fig. 20.1
Transparent blanket covering an infant during induction of general anesthesia previous to the regional block
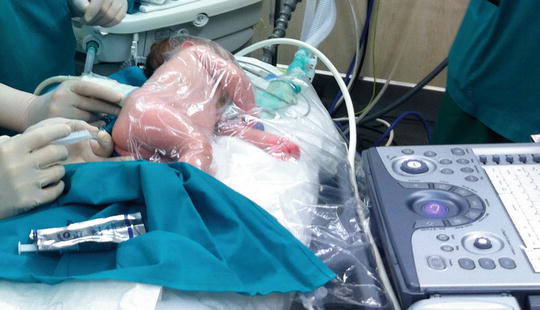
Fig. 20.2
Transparent blanket covering an infant during an ultrasound-assisted caudal block

Fig. 20.3
Warming of ultrasound gel previous to its use in ultrasound-guided pediatric regional anesthesia
Appropriate pediatric regional equipment should always be used. Needles should be marked (1 or 0.5 cm); have an appropriate tip, gauge, and length; and injection extension tubes are recommended (Fig. 20.4). All local anesthetics used in adults may be used in children. The most commonly used local anesthetics are ropivacaine, levobupivacaine, and bupivacaine . Nevertheless, it is now recommended that L-enantiomers are used due to their lower cardiac toxicity compared with bupivacaine . After extravascular injection, the plasma concentration of ropivacaine peaks later than that of bupivacaine , sometimes up to more than 2 h after injection. This delay in the peak plasma concentration of ropivacaine usually reduces the maximum plasma concentration, providing some security in terms of toxicity [70, 71]. Even if the plasma concentration of free and total ropivacaine is higher in the youngest groups of children, plasma concentrations of ropivacaine and its main metabolite (2,6-pipecoloxylidide) are not influenced by the duration of infusion of local anesthetic. The clearance of ropivacaine increases with age but remains unchanged throughout the infusion in each age category. Therefore, ropivacaine seems to be more appropriate, more predictable and safer during continuous infusion for 48–72 h compared with bupivacaine [72].
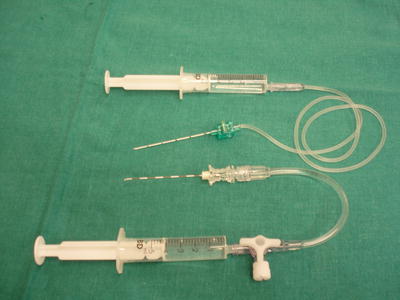

Fig. 20.4
Needles should be of appropriate tip, gauge, and length, and injection extension tubes are recommended
It is the present author’s opinion that regional blocks are indicated in all children without a formal contraindication. True contraindications include coagulopathy, sepsis, or infection at the needle insertion site, true local anesthetic allergy, and refusal by the child or parents. For central blocks, relative contraindications are myelomeningocele, ventriculoperitoneal shunt, and progressive neurologic disease. Risks and benefits in these patients should be carefully considered on an individual basis. For peripheral nerve blocks , a relative contraindication may be the risk of compartment syndrome. In this author’s personal opinion, analgesic regional blocks may be performed only if the surgical team agrees and provided that a dense motor block is not achieved. In these cases, if the surgery has been done with a block, it is imperative to wait for a partial recovery of the motor block (if any) before starting a continuous infusion of local anesthetic in the postoperative period. Any breakthrough or pain out of proportion to that expected should be carefully assessed before increasing the local anesthetic infusion or adding systemic analgesia. Early intramuscular pressure measurement should be available in the facility if regional blocks are to be performed in higher risk patients .
Both central and peripheral blocks should be performed under strict aseptic conditions. Anesthesiologists should follow their hospital’s protocols regarding the use of chlorhexidine or povidone.
In a caudal block, direct visualization of the location of the needle tip with ultrasound is recommended. The spread of the local anesthetic while injected should then be assessed. In an epidural blockade, air loss of resistance techniques should be avoided in pediatric patients because children can develop a life-threatening venous air embolism from small quantities of air, especially in presence of patent foramen ovale (up to 50 % in children younger than 5 years of age). An ultrasound-assisted technique is recommended: the epidural space is found by loss of resistance to saline and subsequently incremental doses of local anesthetic are administered through the catheter while the spread is assessed under ultrasound imaging. As no method of test dosing is infallible, incremental and slow injection is a critical safety measure whenever large volumes of local anesthetics are injected in children. Fixation is to be done with a specific transparent fixation device to allow observation of the catheter or possible signs of infection. Fixation should only be changed if strictly necessary. Catheters should be removed and the tip cultured if the child develops fever >38 °C. Most epidural catheters for postoperative pain relief can be removed after 48–72 h but if the catheter is to be kept in place for more than 48 h tunneling is recommended.
Placement of peripheral nerve catheters is now common in pediatric regional anesthesia [73–77]. Continuous peripheral nerve blocks should be done under strict aseptic conditions and in this author’s opinion mainly for major surgery or pain/rehabilitation therapy. Complications consequence of the technique should be avoided by a very careful procedure performed by skilled pediatric anesthesiologists. Complications derived from infusions and catheter’s care need extensive team training. Personnel from the anesthesia pain team should inspect the catheter daily through a transparent fixation dressing (but only change the dressing if strictly necessary) and control the infusions .
Because drug errors are higher in centers where fewer catheters are managed in the wards, the author of the present chapter recommends thorough staff education programs and medical support to the ward personnel before catheters are managed in a the ward. Intravenous lipid emulsion resuscitation guidelines and intramuscular pressure measurement devices should be available in all locations where continuous local anesthetics are used.
References
1.
Broadman LM, Holt RA: The evidence-based safety of pediatric regional anesthesia and complications. In: Finucane BT (ed.), Complications of regional anesthesia, 2nd ed. Springer, New York 2007, pp. 224–241CrossRef
2.
3.
4.
Fox MA, Webb RK, Singleton R, Ludbrook G, Runciman WB. The Australian incident monitoring study. Problems with regional anaesthesia: an analysis of 2000 incident reports. Anaesth Intensive Care. 1993;21:646–9.PubMed
5.
Zadra N, Giusti F. Il blocco caudale in pediatria. Minerva Anestesiol. 2001;67(Suppl 1):126–31.PubMed
6.
8.
9.
10.
11.
12.
13.
Na EH, Han SJ, Kim MH. Delayed occurrence of spinal arachnoiditis following a caudal block. J Spinal Cord Med. 2011;34:616–9.CrossRefPubMedPubMedCentral
14.
15.
16.
17.
18.
Berde C, Greco C. Pediatric regional anesthesia: drawing inferences on safety from prospective registries and case reports. Anesth Analg. 2012;115:1259–62.CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





